This article contains comparison of key thermal and atomic properties of nitrogen and phosphorus, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Nitrogen vs Phosphorus.
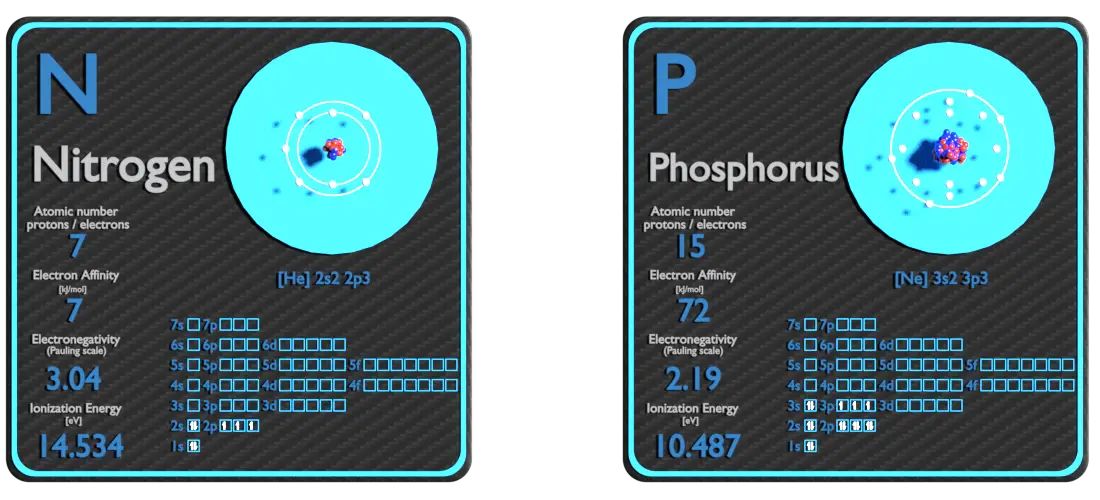
Nitrogen and Phosphorus – About Elements

Source: www.luciteria.com
Nitrogen and Phosphorus – Applications
Nitrogen
Nitrogen in various chemical forms plays a major role in large number of environmental issues. The applications of nitrogen compounds are naturally extremely widely varied due to the huge size of this class: hence, only applications of pure nitrogen itself will be considered here. Two-thirds of nitrogen produced by industry is sold as the gas and the remaining one-third as the liquid. In metallurgy, nitriding is a case hardening process in which the surface nitrogen concentration of a ferrous is increased by diffusion from the surrounding environment to create case-hardened surface. Nitriding produces hard, highly wear-resistant surface (shallow case depths) of product with fair capacity for contact load, good bending fatigue strength and excellent resistance to seizure. Synthetically produced ammonia and nitrates are key industrial fertilisers, and fertiliser nitrates are key pollutants in the eutrophication of water systems. Apart from its use in fertilisers and energy-stores, nitrogen is a constituent of organic compounds as diverse as Kevlar used in high-strength fabric and cyanoacrylate used in superglue.
Phosphorus
Phosphorus is an essential plant nutrient (the most often limiting nutrient, after nitrogen), and the bulk of all phosphorus production is in concentrated phosphoric acids for agriculture fertilisers, containing as much as 70% to 75% P2O5. The vast majority of phosphorus compounds mined are consumed as fertilisers. Phosphate is needed to replace the phosphorus that plants remove from the soil, and its annual demand is rising nearly twice as fast as the growth of the human population. Other applications include organophosphorus compounds in detergents, pesticides, and nerve agents.
Nitrogen and Phosphorus – Comparison in Table
| Element | Nitrogen | Phosphorus |
| Density | 0.00125 g/cm3 | 1.823 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -209.9 °C | 44.1 °C |
| Boiling Point | -195.8 °C | 280 °C |
| Thermal Conductivity | 0.02598 W/mK | 0.235 W/mK |
| Thermal Expansion Coefficient | N/A | N/A |
| Specific Heat | 1.04 J/g K | 0.77 J/g K |
| Heat of Fusion | (N2) 0.7204 kJ/mol | 0.657 kJ/mol |
| Heat of Vaporization | (N2) 5.56 kJ/mol | 51.9 kJ/mol |