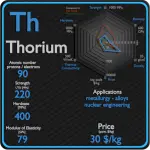
This article contains comparison of key thermal and atomic properties of thorium and uranium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Thorium vs Uranium.
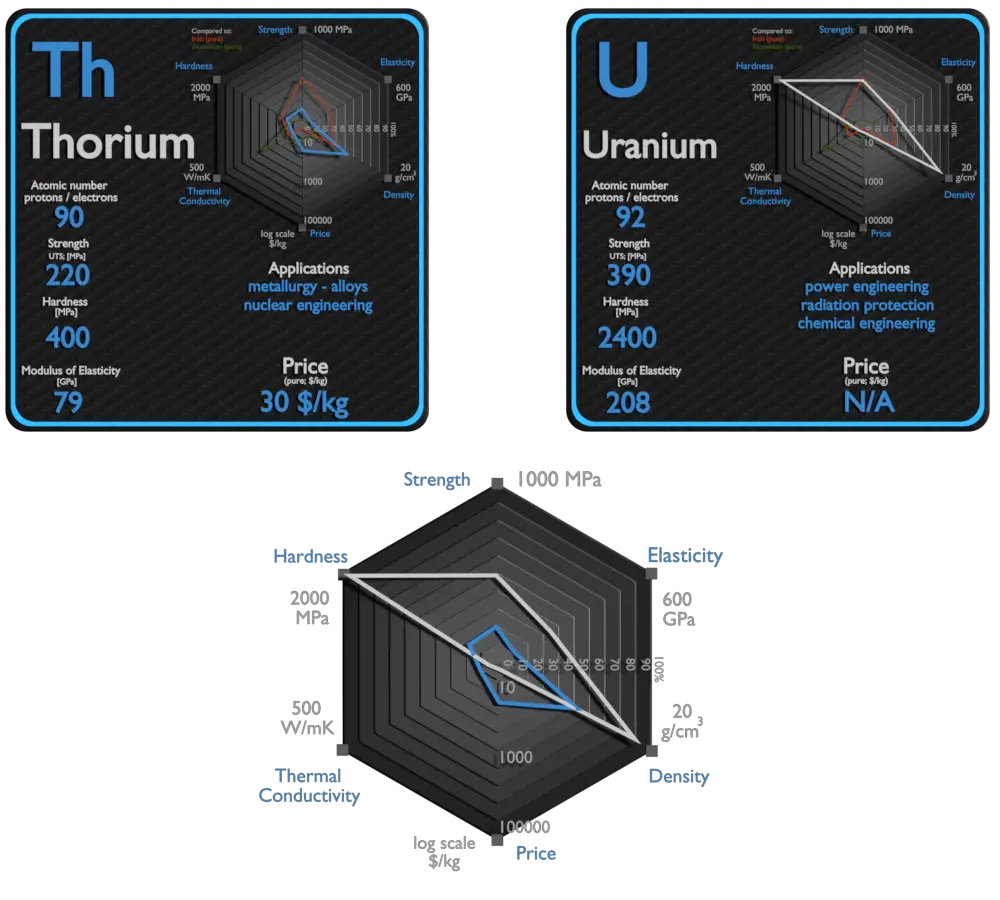
Thorium and Uranium – About Elements


Source: www.luciteria.com
Thorium and Uranium – Applications
Thorium
Most thorium applications use its dioxide (sometimes called “thoria” in the industry), rather than the metal. This compound has a melting point of 3300 °C (6000 °F), the highest of all known oxides; only a few substances have higher melting points.[46] This helps the compound remain solid in a flame, and it considerably increases the brightness of the flame; this is the main reason thorium is used in gas lamp mantles. All substances emit energy (glow) at high temperatures, but the light emitted by thorium is nearly all in the visible spectrum, hence the brightness of thorium mantles. Thorium is an important alloying agent in magnesium, as it imparts greater strength and creep resistance at high temperatures. Thorium oxide is used as an industrial catalyst. Other uses for thorium include heat-resistant ceramics, aircraft engines, and in light bulbs. Thorium can be used as a source of nuclear power. It is about three times as abundant as uranium and about as abundant as lead, and there is probably more energy available from thorium than from both uranium and fossil fuels. 232Th is a fertile isotope. 232Th is not capable of undergoing fission reaction after absorbing thermal neutron, on the other hand 232Th can be fissioned by fast neutron with energy higher than >1MeV. India and China are in the process of developing nuclear power plants with thorium reactors, but this is still a very new technology. Thorium dioxide was formerly added to glass during manufacture to increase the refractive index, producing thoriated glass for use in high-quality camera lenses.
Uranium
The main use of uranium in the civilian sector is to fuel nuclear power plants. One kilogram of uranium-235 can theoretically produce about 20 terajoules of energy, assuming complete fission; as much energy as 1.5 million kilograms (1500 tons) of coal. Typical reactor may contain about 100 tonnes of enriched uranium (i.e. about 113 tonnes of uranium dioxide). This fuel is loaded within, for example, 157 fuel assemblies composed of over 45 000 fuel rods. A common fuel assembly contain energy for approximately 4 years of operation at full power. The removed fuel (spent nuclear fuel) still contains about 96% of reusable material (it must be removed due to decreasing kinf of an assembly). Before (and, occasionally, after) the discovery of radioactivity, uranium was primarily used in small amounts for yellow glass and pottery glazes, such as uranium glass. Uranium is also used by the military to power nuclear submarines and in nuclear weapons. Due to its high density, this material is found in inertial guidance systems and in gyroscopic compasses.[10] Depleted uranium is preferred over similarly dense metals due to its ability to be easily machined and cast as well as its relatively low cost. The main risk of exposure to depleted uranium is chemical poisoning by uranium oxide rather than radioactivity (uranium being only a weak alpha emitter). Depleted uranium is uranium that has much less uranium-235 than natural uranium. It is considerably less radioactive than natural uranium. It is a dense metal that can be used as ballast for ships and counterweights for aircraft. It is also used in ammunition and armour. Depleted uranium can be also used to shield radiation. Depleted uranium is much more effective due to its higher Z. Depleted uranium is used for shielding in portable gamma ray sources. Uranium is used in high-speed steels as an alloying agent to improve strength and toughness. Uranium trioxide (also called uranic oxide) with formula UO3, is an orange-yellow powder and is used as a pigment for ceramics. In glasses it produces a beautiful greenish-yellow “uranium glass”.
Thorium and Uranium – Comparison in Table
| Element | Thorium | Uranium |
| Density | 11.724 g/cm3 | 19.05 g/cm3 |
| Ultimate Tensile Strength | 220 MPa | 390 MPa |
| Yield Strength | 144 MPa | 190 MPa |
| Young’s Modulus of Elasticity | 79 GPa | 208 GPa |
| Mohs Scale | 3 | 6 |
| Brinell Hardness | 400 MPa | 2400 MPa |
| Vickers Hardness | 350 MPa | 1960 MPa |
| Melting Point | 1750 °C | 1132 °C |
| Boiling Point | 4790 °C | 4131 °C |
| Thermal Conductivity | 54 W/mK | 27 W/mK |
| Thermal Expansion Coefficient | 11 µm/mK | 13.9 µm/mK |
| Specific Heat | 0.12 J/g K | 0.12 J/g K |
| Heat of Fusion | 13.8 kJ/mol | 8.52 kJ/mol |
| Heat of Vaporization | 514.4 kJ/mol | 417 kJ/mol |