This article contains comparison of key thermal and atomic properties of argon and krypton, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Argon vs Krypton.
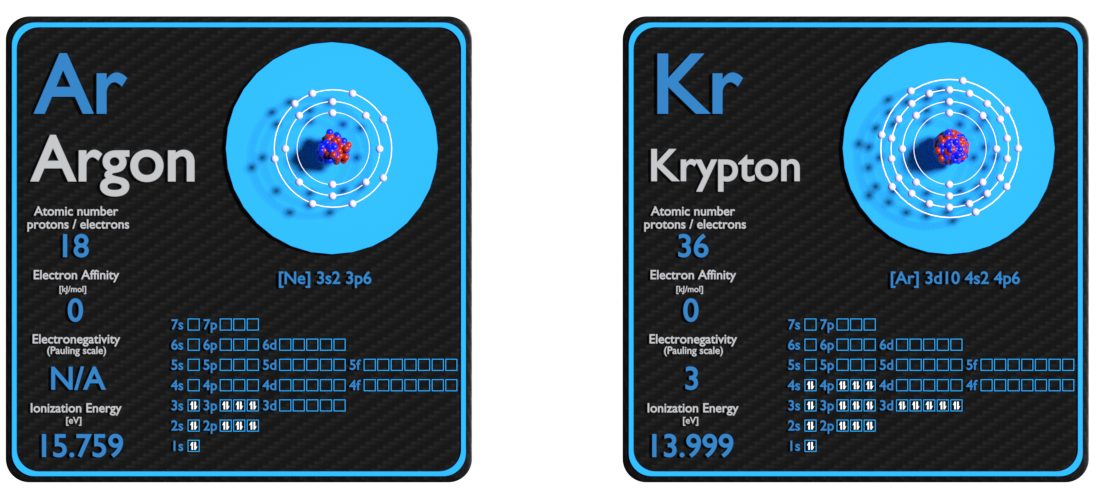
Argon and Krypton – About Elements


Source: www.luciteria.com
Argon and Krypton – Applications
Argon
The major applications of argon include the following: electric lamps as filler gas, welding purpose, discharge tubes, argon lasers and argon-dye lasers. Argon is mostly used as an inert shielding gas in welding and other high-temperature industrial processes where ordinarily unreactive substances become reactive; for example, an argon atmosphere is used in graphite electric furnaces to prevent the graphite from burning. Argon is also used in incandescent, fluorescent lighting, and other gas-discharge tubes.
Krypton
Krypton’s multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed photography. Krypton is also used in MRI/CT techniques.
Argon and Krypton – Comparison in Table
| Element | Argon | Krypton |
| Density | 0.00178 g/cm3 | 0.00375 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -189.2 °C | -157.36 °C |
| Boiling Point | -185.7 °C | -153.22 °C |
| Thermal Conductivity | 0.01772 W/mK | 0.00949 W/mK |
| Thermal Expansion Coefficient | N/A | N/A |
| Specific Heat | 0.52 J/g K | 0.248 J/g K |
| Heat of Fusion | 1.188 kJ/mol | 1.638 kJ/mol |
| Heat of Vaporization | 6.447 kJ/mol | 9.029 kJ/mol |




