This article contains comparison of key thermal and atomic properties of lanthanum and cerium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Lanthanum vs Cerium.
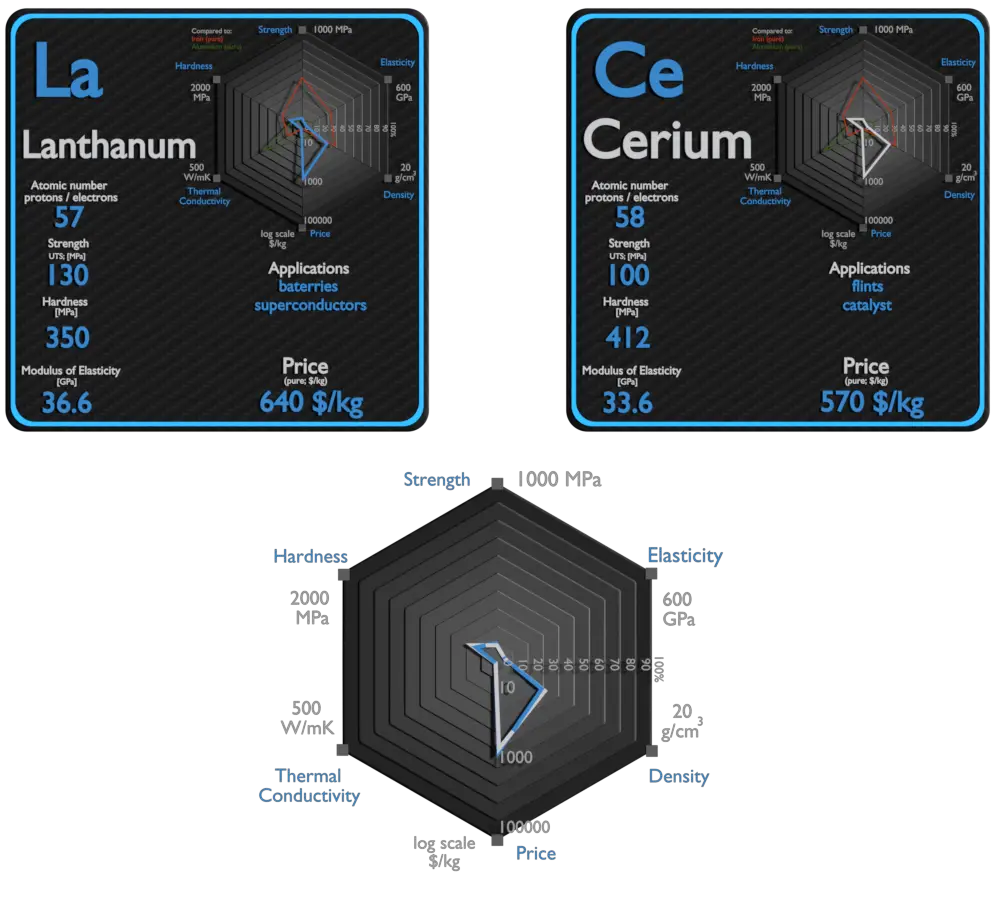
Lanthanum and Cerium – About Elements


Source: www.luciteria.com
Lanthanum and Cerium – Applications
Lanthanum
Lanthanum is not extensively used metal. However, its alloys have a variety of interesting uses. A lanthanum-nickel alloy is used to store hydrogen gas for use in hydrogen-powered vehicles. Lanthanum is also found in the anode of nickel metal hydride batteries (NiMH) used in hybrid cars. Lanthanum is an important component of mischmetal alloy. A typical composition includes approximately 55% cerium, 25% lanthanum, and 15-18% neodymium with other rare earth metals following. The best-known use for this alloy is in ‘flints’ for cigarette lighters.
Cerium
Cerium is an important component of mischmetal alloy. Ferrocerium is a synthetic pyrophoric alloy that produces hot sparks that can reach temperatures of 3,000 °C (5,430 °F) when rapidly oxidized by the process of striking the rod, thereby fragmenting it and exposing those fragments to the oxygen in the air. A typical composition includes approximately 55% cerium, 25% lanthanum, and 15-18% neodymium with other rare earth metals following. The best-known use for this alloy is in ‘flints’ for cigarette lighters. Ceria is the most widely used compound of cerium. The main application of ceria is as a polishing compound, for example in chemical-mechanical planarization (CMP).
Lanthanum and Cerium – Comparison in Table
| Element | Lanthanum | Cerium |
| Density | 6.146 g/cm | 6.689 g/cm3 |
| Ultimate Tensile Strength | 130 MPa | 100 MPa |
| Yield Strength | 125 MPa | 90 MPa |
| Young’s Modulus of Elasticity | 36.6 GPa | 33.6 GPa |
| Mohs Scale | 2.5 | 2.5 |
| Brinell Hardness | 350 MPa | 412 MPa |
| Vickers Hardness | 360 MPa | 300 MPa |
| Melting Point | 920 °C | 798 °C |
| Boiling Point | 3454 °C | 3457 °C |
| Thermal Conductivity | 13 W/mK | 11 W/mK |
| Thermal Expansion Coefficient | 12.1 µm/mK | 6.3 µm/mK |
| Specific Heat | 0.19 J/g K | 0.19 J/g K |
| Heat of Fusion | 6.2 kJ/mol | 5.46 kJ/mol |
| Heat of Vaporization | 414 kJ/mol | 414 kJ/mol |


