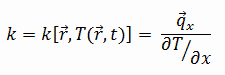Corrosion-resistant alloys, as their name indicates, are alloys with enhanced corrosion resistance. Some ferrous and many non-ferrous metals and alloys are widely used in corrosive environments. In all cases, it strongly depends on certain environment and other conditions. Corrosion-resistant alloys are used for water piping and many chemical and industrial applications. In case of ferrous alloys, we are talking about stainless steels and to some extent about cast irons. But some non-ferrous corrosion-resistant alloys exhibit remarkable corrosion resistance nad therefore they may be used for many special purposes. There are two main reasons why nonferrous materials are preferred over steels and stainless steels for many of these applications. For example, many of the non-ferrous metals and alloys possess much higher resistance to corrosion than available alloy steels and stainless steel grades. Second, a high strength-to-weight ratio or high thermal and electrical conductivity may provide a distinct advantage over a ferrous alloy.
Corrosion-resistant alloys, as their name indicates, are alloys with enhanced corrosion resistance. Some ferrous and many non-ferrous metals and alloys are widely used in corrosive environments. In all cases, it strongly depends on certain environment and other conditions. Corrosion-resistant alloys are used for water piping and many chemical and industrial applications. In case of ferrous alloys, we are talking about stainless steels and to some extent about cast irons. But some non-ferrous corrosion-resistant alloys exhibit remarkable corrosion resistance nad therefore they may be used for many special purposes. There are two main reasons why nonferrous materials are preferred over steels and stainless steels for many of these applications. For example, many of the non-ferrous metals and alloys possess much higher resistance to corrosion than available alloy steels and stainless steel grades. Second, a high strength-to-weight ratio or high thermal and electrical conductivity may provide a distinct advantage over a ferrous alloy.
Thermal Properties of Corrosion-resistant Alloys
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Corrosion-resistant Alloys
Melting point of aluminium bronze – UNS C95400 is around 1030°C.
Melting point of superalloy – Inconel 718 steel is around 1400°C.
Melting point of commercially pure titanium – Grade 2 is around 1660°C.
Melting point of 6061 aluminium alloy is around 600°C.
Melting point of stainless steel – type 304 steel is around 1450°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Corrosion-resistant Alloys
The thermal conductivity of aluminium bronze – UNS C95400 is 59 W/(m.K).
The thermal conductivity of superalloy – Inconel 718 is 6.5 W/(m.K).
The thermal conductivity of commercially pure titanium – Grade 2 is 16 W/(m.K).
The thermal conductivity of 6061 aluminium alloy is 150 W/(m.K).
The thermal conductivity of stainless steel – type 304 is 20 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Thermal Properties of Corrosion-resistant Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.