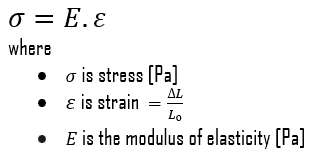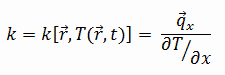Tin is a post-transition metal in group 14 of the periodic table. It is obtained chiefly from the mineral cassiterite, which contains tin dioxide. The first alloy used on a large scale was bronze, made of tin and copper, from as early as 3000 BC. Tin is one of the first metals known to humans, it is nontoxic, soft and pliable, and suitable for cold rolling. Tin resists corrosion, making it an ideal coating for other metals. Tin has a low coefficient of friction, and the addition of alloying elements such as copper, antimony, bismuth, cadmium, and silver increase its hardness. Tin has long been used in alloys with lead as solder. Tin itself has a very low melting point, tin alloyed with lead forms a eutectic mixture at the weight proportion of 61.9% tin and 38.1% lead with melting temperature of 183 °C (361.4 °F). Such solders are primarily used for joining pipes or electric circuits.
Tinplate – Tinning – Hot-dipping – Electroplating
 The largest single application of tin is in the manufacture of tinplate (steel sheet coated with tin), which accounts for approximately 40% of total world tin consumption. Tin bonds readily to iron and steel to prevent corrosion. Tin-plated steel containers are widely used for food preservation, and this forms a large part of the market for metallic tin.
The largest single application of tin is in the manufacture of tinplate (steel sheet coated with tin), which accounts for approximately 40% of total world tin consumption. Tin bonds readily to iron and steel to prevent corrosion. Tin-plated steel containers are widely used for food preservation, and this forms a large part of the market for metallic tin.
Tinning is the process of thinly coating sheets of wrought iron or steel with tin, and the resulting product is known as tinplate. The term is also widely used for the different process of coating a metal with solder before soldering. There are two processes for the tinning of the black plates: hot-dipping and electroplating.
- Hot-dipping. Tinplate made via hot-dipped tin plating is made by cold rolling steel or iron, which is then coated with a thin layer of tin.
- Electroplating. Electroplating is a process that uses an electric current to reduce dissolved metal cations so that they form a thin coherent metal coating on an electrode. The traditional hot-dip method of making tinplate has been largely replaced by electrodeposition of tin on continuous strips of rolled steel.
Solder – Tin – Lead Eutectic Alloy
 Soldering is a technique for joining metals using a filler metal alloy that has a melting temperature less than about 425°C (800°F). Because of this lower temperature and different alloys used as fillers, the metallurgical reaction between filler and work piece is minimal, resulting in a weaker joint. In electronics assembly, the eutectic alloy with 63% tin and 37% lead (or 60/40, which is almost identical in melting point) has been the alloy of choice. This eutectic alloy has melting point lower than those of either tin or lead.
Soldering is a technique for joining metals using a filler metal alloy that has a melting temperature less than about 425°C (800°F). Because of this lower temperature and different alloys used as fillers, the metallurgical reaction between filler and work piece is minimal, resulting in a weaker joint. In electronics assembly, the eutectic alloy with 63% tin and 37% lead (or 60/40, which is almost identical in melting point) has been the alloy of choice. This eutectic alloy has melting point lower than those of either tin or lead.
Tin is an important constituent in solders because it wets and adheres to many common base metals at temperatures considerably below their melting points. Small amounts of various metals, notably antimony and silver, are added to tin-lead solders to increase their strength. 60-40 solder provides strong and reliable joints under a variety of environmental conditions. There are also high-tin solders, which are used for joining parts of electrical apparatuses because their electrical conductivity is higher than that of high-lead solders. These solders are also used where lead may be a hazard, for example, in contact with drinking water or food.
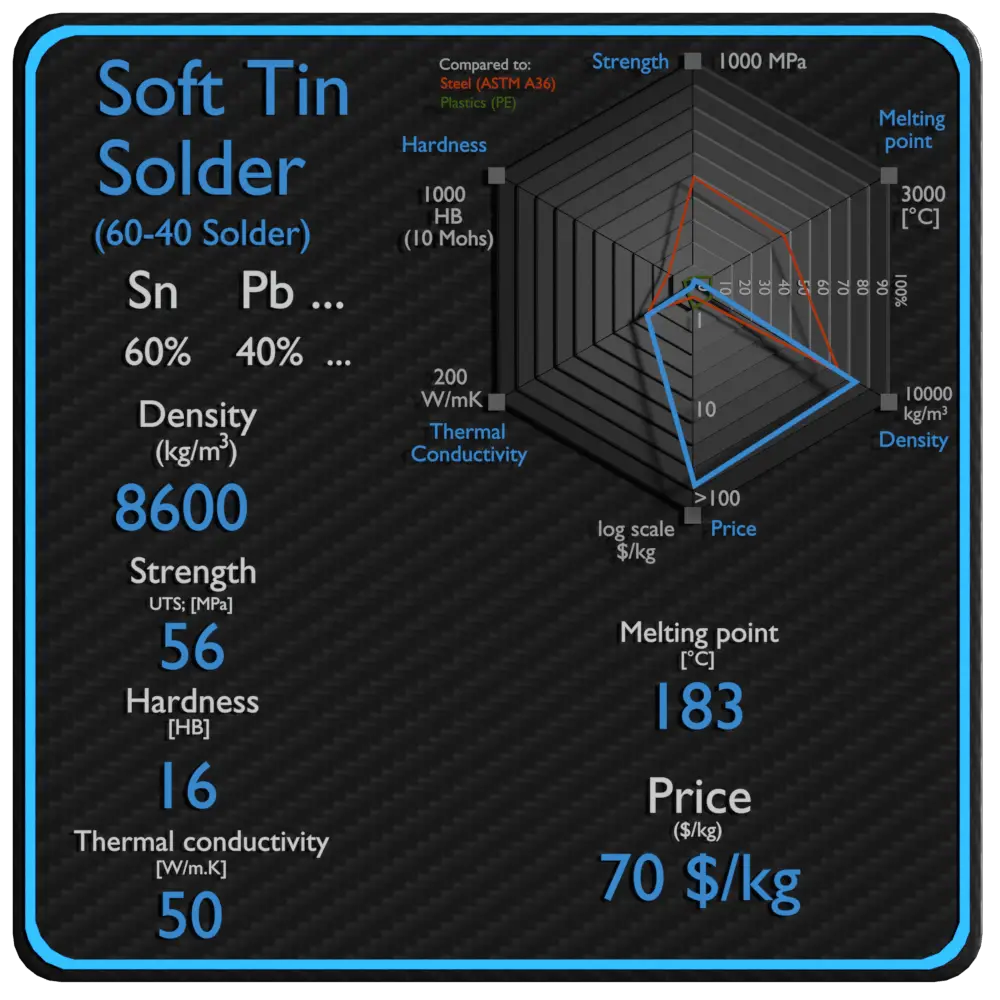
Summary
| Name | Soft Tin Solder |
| Phase at STP | N/A |
| Density | 8600 kg/m3 |
| Ultimate Tensile Strength | 56 MPa |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | 30 GPa |
| Brinell Hardness | 16 BHN |
| Melting Point | 183 °C |
| Thermal Conductivity | 50 W/mK |
| Heat Capacity | 167 J/g K |
| Price | 70 $/kg |
Properties of Soft Solder – 60-40 Solder
Material properties are intensive properties, that means they are independent of the amount of mass and may vary from place to place within the system at any moment. The basis of materials science involves studying the structure of materials, and relating them to their properties (mechanical, electrical etc.). Once a materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and the way in which it has been processed into its final form.
Mechanical Properties of Soft Solder – 60-40 Solder
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial and engineers must take them into account.
Strength of Nickel Alloys
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. Strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
Ultimate tensile strength of soft solder – 60-40 solder depends greatly on the temperature, but for 19°C is about 56 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of soft solder – 60-40 solder is about 30 GPa.
The Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to a limiting stress, a body will be able to recover its dimensions on removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position. All the atoms are displaced the same amount and still maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions and no permanent deformation occurs. According to the Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
Hardness of Soft Solder – 60-40 Solder
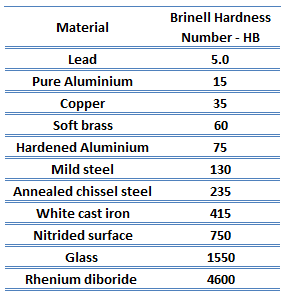
Brinell hardness of soft solder – 60-40 solder approximately 16 HB.
Rockwell hardness test is one of the most common indentation hardness tests, that has been developed for hardness testing. In contrast to Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position. The major load is applied, then removed while still maintaining the minor load. The difference between depth of penetration before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Soft Solder – 60-40 Solder
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Soft Solder – 60-40 Solder
Melting point of soft solder – 60-40 solder is around 183°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Soft Solder – 60-40 Solder
The thermal conductivity of soft solder – 60-40 solder is 50 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Electrical Resisitivity of Soft Solder – 60-40 Solder
Electrical resistivity of soft solder – 60-40 solder is 150 x 10−9 Ω·m.
Electrical resistivity and its converse, electrical conductivity, is a fundamental property of a material that quantifies how strongly it resists or conducts the flow of electric current. A low resistivity indicates a material that readily allows the flow of electric current. The symbol of resistivity is usually the Greek letter ρ (rho). The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). Note that, electrical resistivity is not the same as electrical resistance. Electrical resistance is expressed in Ohms. While resistivity is a material property, resistance is the property of an object.
[/lgc_column]We hope, this article, Tin Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.