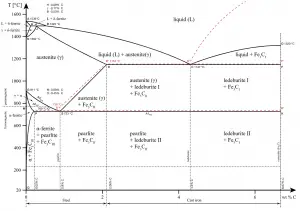
As was written, cast irons are one of the most complex alloys used in industry. Because of the higher carbon content, the structure of cast iron, as opposed to that of steel, exhibits a carbon-rich phase. Depending primarily on composition, cooling rate, and melt treatment, the carbon-rich phase can solidify with formation of either a stable (austenite-graphite) or a metastable (austenite-Fe3C) eutectic.
With a lower silicon content (containing less than 1.0 wt% Si – graphitizing agent) and faster cooling rate, the carbon in cast iron precipitates out of the melt as the metastable phase cementite, Fe3C, rather than graphite. The product of this solidification is known as white cast iron (also known as chilled irons). White cast irons are hard, brittle, and unmachinable, while gray irons with softer graphite are reasonably strong and machinable. A fracture surface of this alloy has a white appearance, and thus it is termed white cast iron. It is difficult to cool thick castings fast enough to solidify the melt as white cast iron all the way through. However, rapid cooling can be used to solidify a shell of white cast iron, after which the remainder cools more slowly to form a core of gray cast iron. This type of casting, sometimes referred to as a “chilled casting” has a harder outer surface and a tougher inner core.
White iron is too brittle for use in many structural components, but with good hardness and abrasion resistance and relatively low cost, it finds use in such applications where wear resistance is desirable, such as on the teeth of excavators, impellers and volutes of slurry pumps, shell liners and lifter bars in ball mills.
For example, Ni-Cr-HC martensitic white cast iron (Nickel-Chrome-High Carbon Alloy), ASTM A532 Class 1 Type A, is martensitic white cast iron, in which nickel is the primary alloying element because, at levels of 3 to 5%, it is effective in suppressing the transformation of the austenite matrix to pearlite, thus ensuring that a hard, martensitic structure will develop upon cooling inthe mold. This material may also be called Ni-Hard 1. Ni-Hard 1 is an abrasion resistant material used in applications where impact is also a concern as the wear mechanism.
We hope, this article, Composition of White Cast Iron, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.
