
Pure zirconium is a lustrous, grey-white, strong transition metal that resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium and its alloys are widely used as a cladding for nuclear reactor fuels. Zirconium alloyed with niobium or tin has excellent corrosion properties. The high corrosion resistance of zirconium alloys results from the natural formation of a dense stable oxide on the surface of the metal. This film is self healing, it continues to grow slowly at temperatures up to approximately 550 °C (1020 °F), and it remains tightly adherent. The desired property of these alloys is also a low neutron-capture cross-section. The disadvantages of zirconium are low strength properties and low heat resistance, which can be eliminated, for example, by alloying with niobium.
Composition of Zirconium Alloys
- Zirconium – Niobium Alloys. Zirconium alloys with niobium are used as claddings of fuel elements of VVER and RBMK reactors. These alloys are the basis material of assembly channel of RBMK reactor. The Zr + 1% Nb alloy of type N-1 E-110 is used for fuel element claddings, the Zr + 2.5% Nb alloy of type E-125 is applied for tubes of assembly channels.
- Zirconium – Tin Alloys. Zirconium alloys, in which tin is the basic alloying element, provides improvement of their mechanical properties, have a wide distribution in the USA. A common subgroup has the trade mark Zircaloy. In case of zirconium-tin alloys, the decrease of corrosion resistance in water and steam is taken place that resulted in the need for additional alloying.
The cladding material for the new 17×17 fuel designs is based also on the zirconium-niobium alloys (e.g. Optimized ZIRLO material), which has been demonstrated to have improved corrosion resistance compared with prior fuel cladding materials. The optimized tin level provides a reduced corrosion rate while maintaining the benefits of mechanical strength and resistance to accelerated corrosion from abnormal chemistry conditions.
Production of Zirconium
The production of zirconium metal requires special techniques due to the particular chemical properties of zirconium. Most Zr metal is produced from zircon (ZrSiO4) by the reduction of the zirconium chloride with magnesium metal in the Kroll process. The key feature of the Kroll process is reduction of zirconium chloride to metallic zirconium by magnesium. Commercial non-nuclear grade zirconium typically contains 1–5% of hafnium, whose neutron absorption cross-section is 600x that of zirconium. Hafnium must therefore be almost entirely removed (reduced to < 0.02% of the alloy) for reactor applications.
Zirconium Alloys in Nuclear Industry
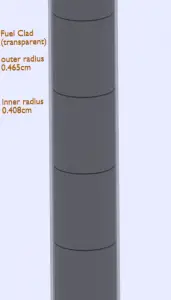
Fuel cladding is the outer layer of the fuel rods, standing between the reactor coolant and the nuclear fuel (i.e. fuel pellets). It is made of a corrosion-resistant material with low absorption cross section for thermal neutrons (~ 0.18 × 10–24 cm2), usually zirconium alloy. The fuel cladding typically has an inner radius of rZr,2 = 0.408 cm and outer radius rZr,1 = 0.465 cm. In comparison to fuel pellet, there is almost no heat generation in the fuel cladding (cladding is slightly heated by radiation). All heat generated in the fuel must be transferred via conduction through the cladding and therefore the inner surface is hotter than the outer surface.
A typical composition of nuclear-grade zirconium alloys is more than 95 weight percent zirconium and less than 2% of tin, niobium, iron, chromium, nickel and other metals, which are added to improve mechanical properties and corrosion resistance. The most commonly used alloy, to date, in PWRs, has been Zircaloy 4, however currently this is being replaced by new zirconium–niobium-based alloys, exhibiting better corrosion resistance. The maximum temperature, at which zirconium alloys can be used in water cooled reactors, depends on their corrosion resistance. The most common zirconium alloys, Zircaloy-2 and Zircaloy-4, contain the strong α stabilizers tin and oxygen, plus the β stabilizers iron, chromium, and nickel. Alloys of type Zircalloy, in which tin is the basic alloying element that provides improvement of their mechanical properties, have a wide distribution in the world. However in this case, the decrease of corrosion resistance in water and steam is taken place that resulted in the need for additional alloying. The improvement brought about by the additive niobium probably involves a different mechanism. High corrosion resistance of niobium alloyed metals in water and steam at temperatures of 400–550°C is caused by their ability to passivation with formation of protective films.
We hope, this article, Composition of Zirconium Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.