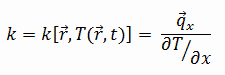Zirconium and its alloys are widely used as a cladding for nuclear reactor fuels. Zirconium alloyed with niobium or tin has excellent corrosion properties. The high corrosion resistance of zirconium alloys results from the natural formation of a dense stable oxide on the surface of the metal. This film is self healing, it continues to grow slowly at temperatures up to approximately 550 °C (1020 °F), and it remains tightly adherent. The desired property of these alloys is also a low neutron-capture cross-section. The disadvantages of zirconium are low strength properties and low heat resistance, which can be eliminated, for example, by alloying with niobium.
- Zirconium – Niobium Alloys. Zirconium alloys with niobium are used as claddings of fuel elements of VVER and RBMK reactors. These alloys are the basis material of assembly channel of RBMK reactor. The Zr + 1% Nb alloy of type N-1 E-110 is used for fuel element claddings, the Zr + 2.5% Nb alloy of type E-125 is applied for tubes of assembly channels.
- Zirconium – Tin Alloys. Zirconium alloys, in which tin is the basic alloying element, provides improvement of their mechanical properties, have a wide distribution in the USA. A common subgroup has the trade mark Zircaloy. In case of zirconium-tin alloys, the decrease of corrosion resistance in water and steam is taken place that resulted in the need for additional alloying.
A typical composition of nuclear-grade zirconium alloys is more than 95 weight percent zirconium and less than 2% of tin, niobium, iron, chromium, nickel and other metals, which are added to improve mechanical properties and corrosion resistance. The most commonly used alloy, to date, in PWRs, has been Zircaloy 4, however currently this is being replaced by new zirconium–niobium-based alloys, exhibiting better corrosion resistance. The maximum temperature, at which zirconium alloys can be used in water cooled reactors, depends on their corrosion resistance. The most common zirconium alloys, Zircaloy-2 and Zircaloy-4, contain the strong α stabilizers tin and oxygen, plus the β stabilizers iron, chromium, and nickel. Alloys of type Zircalloy, in which tin is the basic alloying element that provides improvement of their mechanical properties, have a wide distribution in the world. However in this case, the decrease of corrosion resistance in water and steam is taken place that resulted in the need for additional alloying. The improvement brought about by the additive niobium probably involves a different mechanism. High corrosion resistance of niobium alloyed metals in water and steam at temperatures of 400–550°C is caused by their ability to passivation with formation of protective films.
Oxidation of Zirconium Alloys
The oxidation of zirconium alloys is one of the most studied processes in all of the nuclear industry. Oxidative reaction of zirconium with water releases hydrogen gas, which partly diffuses into the alloy and forms zirconium hydrides. The hydrides are less dense and are weaker mechanically than the alloy; their formation results in blistering and cracking of the cladding – a phenomenon known as hydrogen embrittlement. While many of these reports are written to address the reaction of fuel and steam with zirconium alloys in the case of a nuclear accident, there are still a substantial number of reports dealing with the oxidation of zirconium alloys at moderate temperatures of about 800 K and below.
Zr + 2H2O→ZrO2 + 2H2
At high temperatures, the exothermic reaction of Zr-base alloys with steam is much more intensive and hazardous for the safety of nuclear power plants during accidents like a loss-of-coolant accident (LOCA). The main problem of high temperature oxidation is that zirconium cladding rapidly reacts with water steam at high temperature. The oxidation kinetics of relevant zirconium alloys appears to be parabolic in the temperature range of 1000-1500°C for many Zr-based alloys.
Thermal Properties of Zirconium Alloy – Zircaloy – 4
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Zirconium Alloy – Zircaloy – 4
Melting point of zirconium alloy – Zircaloy – 4 is around 1850°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Zirconium Alloy – Zircaloy – 4
Zirconium alloys have lower thermal conductivity (about 18 W/m.K) than pure zirconium metal (about 22 W/m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Thermal Properties of Zirconium Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.