 Corrosion-resistant alloys, as their name indicates, are alloys with enhanced corrosion resistance. Some ferrous and many non-ferrous metals and alloys are widely used in corrosive environments. In all cases, it strongly depends on certain environment and other conditions. Corrosion-resistant alloys are used for water piping and many chemical and industrial applications. In case of ferrous alloys, we are talking about stainless steels and to some extent about cast irons. But some non-ferrous corrosion-resistant alloys exhibit remarkable corrosion resistance nad therefore they may be used for many special purposes. There are two main reasons why nonferrous materials are preferred over steels and stainless steels for many of these applications. For example, many of the non-ferrous metals and alloys possess much higher resistance to corrosion than available alloy steels and stainless steel grades. Second, a high strength-to-weight ratio or high thermal and electrical conductivity may provide a distinct advantage over a ferrous alloy.
Corrosion-resistant alloys, as their name indicates, are alloys with enhanced corrosion resistance. Some ferrous and many non-ferrous metals and alloys are widely used in corrosive environments. In all cases, it strongly depends on certain environment and other conditions. Corrosion-resistant alloys are used for water piping and many chemical and industrial applications. In case of ferrous alloys, we are talking about stainless steels and to some extent about cast irons. But some non-ferrous corrosion-resistant alloys exhibit remarkable corrosion resistance nad therefore they may be used for many special purposes. There are two main reasons why nonferrous materials are preferred over steels and stainless steels for many of these applications. For example, many of the non-ferrous metals and alloys possess much higher resistance to corrosion than available alloy steels and stainless steel grades. Second, a high strength-to-weight ratio or high thermal and electrical conductivity may provide a distinct advantage over a ferrous alloy.
Density of Corrosion-resistant Alloys
Density of typical aluminium bronze is 7.45 g/cm3 (UNS C95400).
Density of typical superalloy is 8.22 g/cm3 (Inconel 718).
Density of typical titanium alloy is 4.51 g/cm3 (Grade 2).
Density of typical aluminium alloy is 2.7 g/cm3 (6061 alloy).
Density of typical stainless steel is 8.0 g/cm3 (304 steel).
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
In words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/m3). The Standard English unit is pounds mass per cubic foot (lbm/ft3).
Since the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance, it is obvious, the density of a substance strongly depends on its atomic mass and also on the atomic number density (N; atoms/cm3),
- Atomic Weight. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Therefore it is determined by the mass number (number of protons and neutrons).
- Atomic Number Density. The atomic number density (N; atoms/cm3), which is associated with atomic radii, is the number of atoms of a given type per unit volume (V; cm3) of the material. The atomic number density (N; atoms/cm3) of a pure material having atomic or molecular weight (M; grams/mol) and the material density (⍴; gram/cm3) is easily computed from the following equation using Avogadro’s number (NA = 6.022×1023 atoms or molecules per mole):
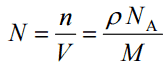
- Crystal Structure. Density of crystalline substance is significantly affected by its crystal structure. FCC structure, along with its hexagonal relative (hcp), has the most efficient packing factor (74%). Metals containing FCC structures include austenite, aluminum, copper, lead, silver, gold, nickel, platinum, and thorium.
We hope, this article, Density of Corrosion-resistant Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.