Corrosion is the deterioration of a material due to chemical interaction with its environment. It is natural process in which metals convert its structure into a more chemically-stable form such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon, since it is associated with mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion. Even the noble metals, such as gold, are subject to corrosive attack in some environments.
Galvanic Corrosion
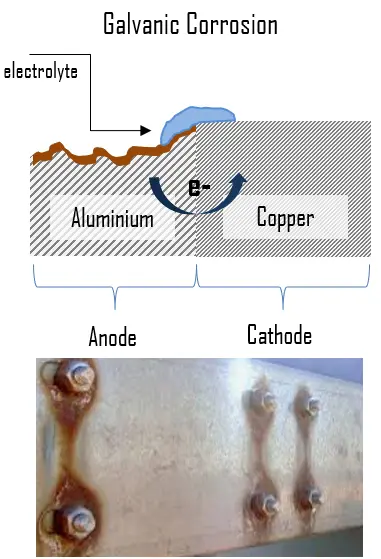 Galvanic corrosion occurs when two dissimilar metals are immersed in a conductive solution in presence of some potential difference and there is a flow of electron between the metals. It may also take place with one metal with heterogeneities (dissimilarities) (for example, impurity inclusions, grains of different sizes, difference in composition of grains, or differences in mechanical stress). The metal which is less corrosive resistant becomes anode and metal with more corrosive resistance become cathode. The corrosion of the less corrosive resistance is usually increased and attack on more resistant material is decreased. A difference in electrical potential exists between the different metals and serves as the driving force for electrical current flow through the corrodant or electrolyte.
Galvanic corrosion occurs when two dissimilar metals are immersed in a conductive solution in presence of some potential difference and there is a flow of electron between the metals. It may also take place with one metal with heterogeneities (dissimilarities) (for example, impurity inclusions, grains of different sizes, difference in composition of grains, or differences in mechanical stress). The metal which is less corrosive resistant becomes anode and metal with more corrosive resistance become cathode. The corrosion of the less corrosive resistance is usually increased and attack on more resistant material is decreased. A difference in electrical potential exists between the different metals and serves as the driving force for electrical current flow through the corrodant or electrolyte.
Galvanic corrosion occurs only if the following conditions are met:
- Two different metals must be present
- The two metals must be in contact, or an electrically conductive path between the two must be present
- There must be an electrically conductive path for the ions to move from the “anode” to the “cathode”
If any of these conditions is not satisfied, galvanic corrosion is not likely to take place.
Galvanic corrosion only causes deterioration of one of the metals. The stronger, more noble one is cathodic (positive) and protected. This is the mechanism of galvanic anodes, which are the main component of a galvanic cathodic protection (CP) system used to protect buried or submerged metal structures from corrosion. In some instances, galvanic corrosion can be helpful.
We hope, this article, Galvanic Corrosion, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.