This article contains comparison of key thermal and atomic properties of rubidium and caesium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Rubidium vs Caesium.
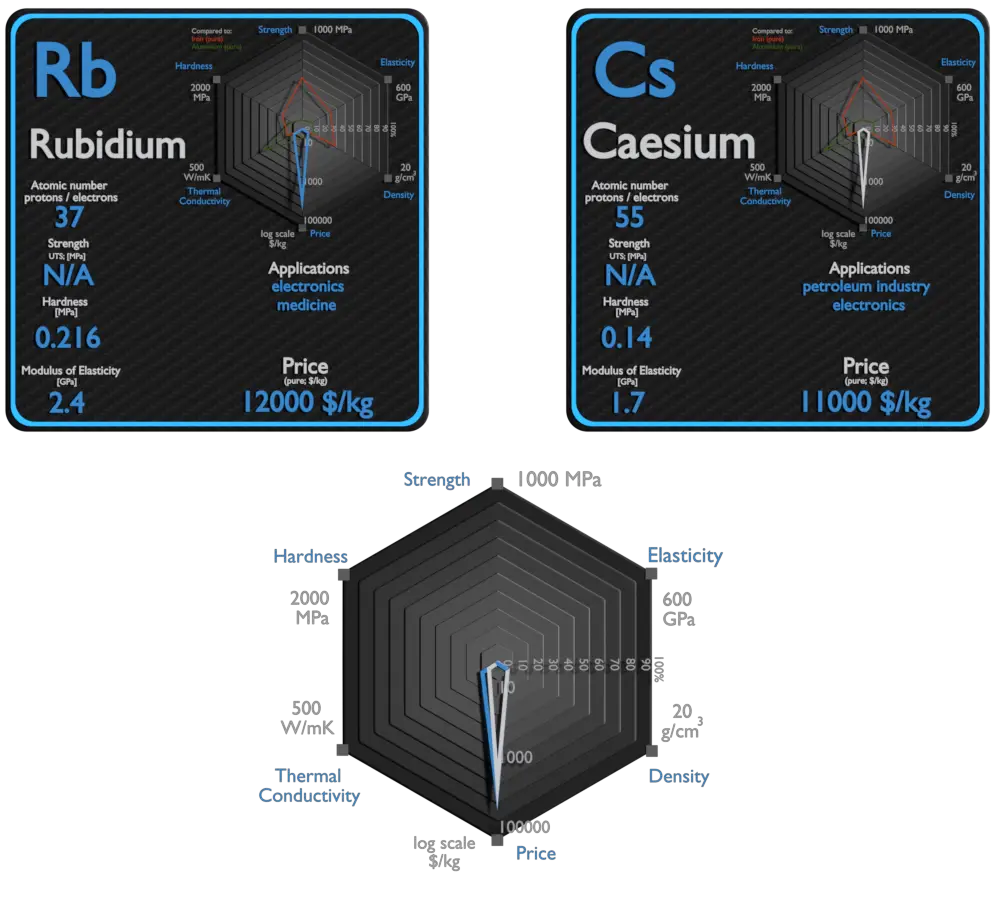
Rubidium and Caesium – About Elements


Source: www.luciteria.com
Rubidium and Caesium – Applications
Rubidium
The photoemissive property of rubidium, which is that of a surface emitting free electrons when impinged upon by electromagnetic radiation, makes possible the following applications. A rubidium-tellurium photoemissive surface is used in photoelectric cells, which are incorporated in a variety of electronic detection and activation devices. It is sensitive to a wide spectrum of radiation from the mid-ultraviolet through the visible into the near-infrared. A rubidium-cesium-antimony coating is commonly applied to the photocathodes of photomultiplier tubes. Rubidium-82 is used for positron emission tomography.
Caesium
The largest present-day use of nonradioactive caesium is in caesium formate drilling fluids for the extractive oil industry. They are also used to make special optical glass, as a catalyst promoter, in vacuum tubes and in radiation monitoring equipment. One of its most important uses is in the ‘caesium clock’ (atomic clock). These clocks are a vital part of the internetand mobile phone networks, as well as Global Positioning System (GPS) satellites. Caesium-137 is a radioisotope commonly used as a gamma-emitter in industrial applications.
Rubidium and Caesium – Comparison in Table
| Element | Rubidium | Caesium |
| Density | 1.532 g/cm3 | 1.879 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | 2.4 GPa | 1.7 GPa |
| Mohs Scale | 0.3 | 0.2 |
| Brinell Hardness | 0.216 MPa | 0.14 MPa |
| Vickers Hardness | N/A | N/A |
| Melting Point | 39.31 °C | 28.4 °C |
| Boiling Point | 688 °C | 669 °C |
| Thermal Conductivity | 58.2 W/mK | 36 W/mK |
| Thermal Expansion Coefficient | 90 µm/mK | 97 µm/mK |
| Specific Heat | 0.363 J/g K | 0.24 J/g K |
| Heat of Fusion | 2.192 kJ/mol | 2.092 kJ/mol |
| Heat of Vaporization | 72.216 kJ/mol | 67.74 kJ/mol |




