This article contains comparison of key thermal and atomic properties of rubidium and strontium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Rubidium vs Strontium.
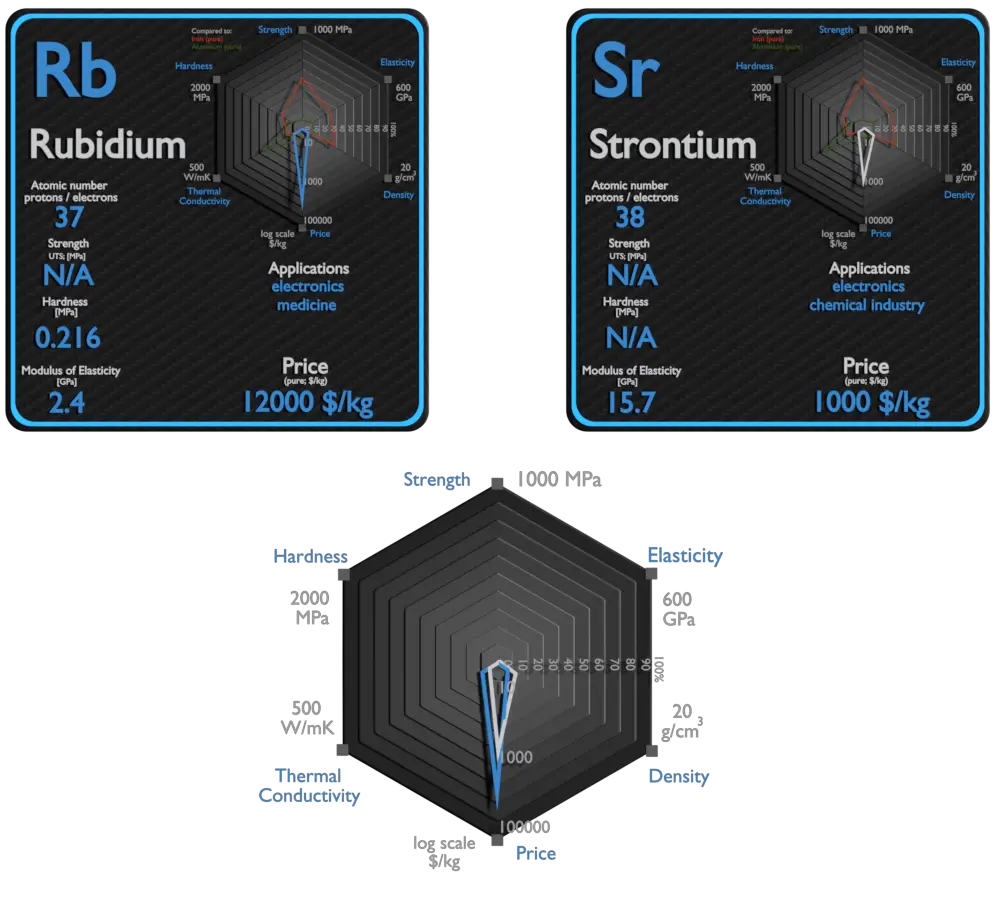
Rubidium and Strontium – About Elements


Source: www.luciteria.com
Rubidium and Strontium – Applications
Rubidium
The photoemissive property of rubidium, which is that of a surface emitting free electrons when impinged upon by electromagnetic radiation, makes possible the following applications. A rubidium-tellurium photoemissive surface is used in photoelectric cells, which are incorporated in a variety of electronic detection and activation devices. It is sensitive to a wide spectrum of radiation from the mid-ultraviolet through the visible into the near-infrared. A rubidium-cesium-antimony coating is commonly applied to the photocathodes of photomultiplier tubes. Rubidium-82 is used for positron emission tomography.
Strontium
Consuming 75% of production, the primary use for strontium was in glass for colour television cathode ray tubes. Strontium is best known for the brilliant reds its salts give to fireworks and flares. It is also used in producing ferrite magnets and refining zinc. Modern ‘glow-in-the-dark’ paints and plastics contain strontium aluminate. They absorb light during the day and release it slowly for hours afterwards. The isotope Sr90 has a half-life of 28 years and is one of the well-known high-energy beta emitters. Hence, it is employed in systems for nuclear auxiliary power (SNAP) devices, which find potential applications in remote weather stations, space vehicles, navigational buoys, etc.
Rubidium and Strontium – Comparison in Table
| Element | Rubidium | Strontium |
| Density | 1.532 g/cm3 | 2.63 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | 2.4 GPa | 15.7 GPa |
| Mohs Scale | 0.3 | 1.8 |
| Brinell Hardness | 0.216 MPa | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | 39.31 °C | 777 °C |
| Boiling Point | 688 °C | 1382 °C |
| Thermal Conductivity | 58.2 W/mK | 35.3 W/mK |
| Thermal Expansion Coefficient | 90 µm/mK | 22.5 µm/mK |
| Specific Heat | 0.363 J/g K | 0.3 J/g K |
| Heat of Fusion | 2.192 kJ/mol | 8.3 kJ/mol |
| Heat of Vaporization | 72.216 kJ/mol | 144 kJ/mol |



