This article contains comparison of key thermal and atomic properties of silicon and germanium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Silicon vs Germanium.
![]()
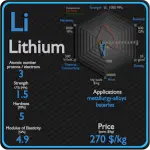
Silicon and Germanium – About Elements
![]()

Source: www.luciteria.com
Silicon and Germanium – Applications
Silicon
Most silicon is used industrially without being purified, and indeed, often with comparatively little processing from its natural form. Silicon is a vital ingredient in aluminum, steel, and iron alloys. It is added as a fluxing agent for copper alloys. In the form of clay and sand, it is used to manufacture bricks and concrete; it is a valuable refractory material for high-temperature work, for example, molding sands for castings in foundry applications. Silica is used to make fire brick, a type of ceramic. Silicate minerals are also in whiteware ceramics, an important class of products usually containing various types of fired clay minerals (natural aluminium phyllosilicates). An example is porcelain, which is based on the silicate mineral kaolinite. Traditional glass (silica-based soda-lime glass) also functions in many of the same ways, and also is used for windows and containers. Hyperpure silicon metal and doped hyperpure silicon (doping with boron, phosphorous, gallium, or arsenic) are used in solar cells, transistors and semiconductors.
Germanium
In gamma spectroscopy, germanium is preferred due to its atomic number being much higher than silicon and which increases the probability of gamma ray interaction. Moreover, germanium has lower average energy necessary to create an electron-hole pair, which is 3.6 eV for silicon and 2.9 eV for germanium. This also provides the latter a better resolution in energy. A large, clean and almost perfect germanium semiconductor is ideal as a counter for radioactivity. However, it is difficult and expensive to make large crystals with sufficient purity. On the other hand, in order to achieve maximum efficiency the detectors must operate at the very low temperatures of liquid nitrogen (-196°C), because at room temperatures the noise caused by thermal excitation is very high. Since germanium detectors produce the highest resolution commonly available today, they are used to measure radiation in a variety of applications including personnel and environmental monitoring for radioactive contamination, medical applications, radiometric assay, nuclear security and nuclear plant safety.
Silicon and Germanium – Comparison in Table
| Element | Silicon | Germanium |
| Density | 2.33 g/cm3 | 5.323 g/cm3 |
| Ultimate Tensile Strength | 170 MPa | 135 MPa |
| Yield Strength | 165 MPa | 135 MPa |
| Young’s Modulus of Elasticity | 150 GPa | 103 GPa |
| Mohs Scale | 7 | 6 |
| Brinell Hardness | 2300 MPa | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | 1410 °C | 938.3 °C |
| Boiling Point | 3265 °C | 2820 °C |
| Thermal Conductivity | 148 W/mK | 59.9 W/mK |
| Thermal Expansion Coefficient | 2.6 µm/mK | 6 µm/mK |
| Specific Heat | 0.71 J/g K | 0.32 J/g K |
| Heat of Fusion | 50.55 kJ/mol | 36.94 kJ/mol |
| Heat of Vaporization | 384.22 kJ/mol | 330.9 kJ/mol |