This article contains comparison of key thermal and atomic properties of tin and lead, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Tin vs Lead.
Tin and Lead – About Elements


Source: www.luciteria.com
Tin and Lead – Applications
Tin
The largest single application of tin is in the manufacture of tinplate (steel sheet coated with tin), which accounts for approximately 40% of total world tin consumption. Tin bonds readily to iron and steel to prevent corrosion. Tin-plated steel containers are widely used for food preservation, and this forms a large part of the market for metallic tin. Tinning is the process of thinly coating sheets of wrought iron or steel with tin, and the resulting product is known as tinplate. The term is also widely used for the different process of coating a metal with solder before soldering. There are two processes for the tinning of the black plates: hot-dipping and electroplating.
Lead
Lead metal has several useful mechanical properties, including high density, low melting point, ductility, and relative inertness. Lead is widely used for car batteries, pigments, ammunition, cable sheathing, weights for lifting, weight belts for diving, lead crystal glass, radiation protection and in some solders. The largest use of lead in the early 21st century is in lead–acid batteries. The lead in batteries undergoes no direct contact with humans, so there are fewer toxicity concerns. Lead is used in high voltage power cables as sheathing material to prevent water diffusion into insulation; this use is decreasing as lead is being phased out. A lead is widely used as a gamma shield. Major advantage of lead shield is in its compactness due to its higher density. On the other hand depleted uranium is much more effective due to its higher Z. Depleted uranium is used for shielding in portable gamma ray sources.
Tin and Lead – Comparison in Table
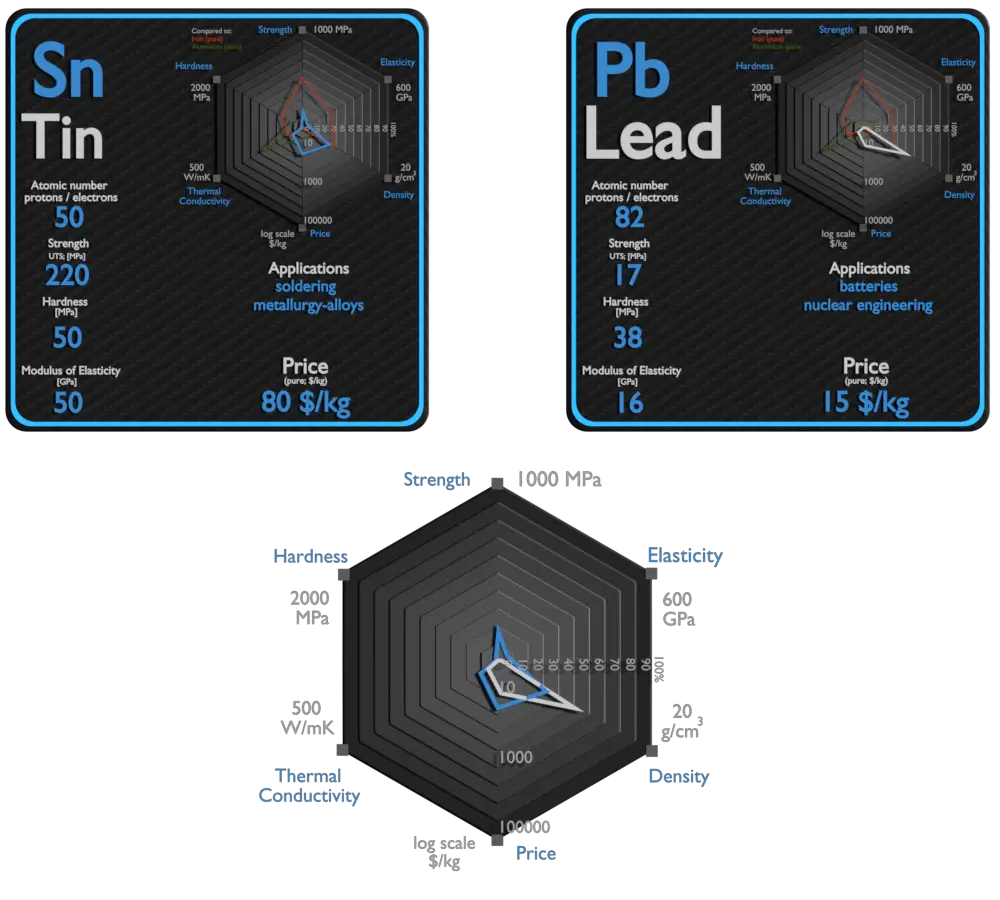
| Element | Tin | Lead |
| Density | 7.31 g/cm3 | 11.34 g/cm3 |
| Ultimate Tensile Strength | 220 MPa | 17 MPa |
| Yield Strength | N/A | 5.5 MPa |
| Young’s Modulus of Elasticity | 50 GPa | 16 GPa |
| Mohs Scale | 1.65 | 1.5 |
| Brinell Hardness | 50 MPa | 38 MPa |
| Vickers Hardness | N/A | N/A |
| Melting Point | 231.93 °C | 327.5 °C |
| Boiling Point | 2602 °C | 1740 °C |
| Thermal Conductivity | 67 W/mK | 35 W/mK |
| Thermal Expansion Coefficient | 22 µm/mK | 28.9 µm/mK |
| Specific Heat | 0.227 J/g K | 0.13 J/g K |
| Heat of Fusion | 7.029 kJ/mol | 4.799 kJ/mol |
| Heat of Vaporization | 295.8 kJ/mol | 177.7 kJ/mol |










