This article contains comparison of key thermal and atomic properties of tin and antimony, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Tin vs Antimony.
Tin and Antimony – About Elements


Source: www.luciteria.com
Tin and Antimony – Applications
Tin
The largest single application of tin is in the manufacture of tinplate (steel sheet coated with tin), which accounts for approximately 40% of total world tin consumption. Tin bonds readily to iron and steel to prevent corrosion. Tin-plated steel containers are widely used for food preservation, and this forms a large part of the market for metallic tin. Tinning is the process of thinly coating sheets of wrought iron or steel with tin, and the resulting product is known as tinplate. The term is also widely used for the different process of coating a metal with solder before soldering. There are two processes for the tinning of the black plates: hot-dipping and electroplating.
Antimony
The largest applications for metallic antimony are an alloy with lead and tin and the lead antimony plates in lead–acid batteries. Alloys of lead and tin with antimony have improved properties for solders, bullets, and plain bearings. Antimony can be used in fire retardants for many commercial and domestic products. Antimony trichloride is used in the manufacturing flame-proofing compounds as well as paints, ceramic enamels, glass and pottery. Other uses include ball bearings and mixing with alloys with percentages ranging from 1 to 20 greatly increasing the hardness and mechanical strength of the lead. The capability to strengthen already strong alloys is its largest and most widespread use.
Tin and Antimony – Comparison in Table
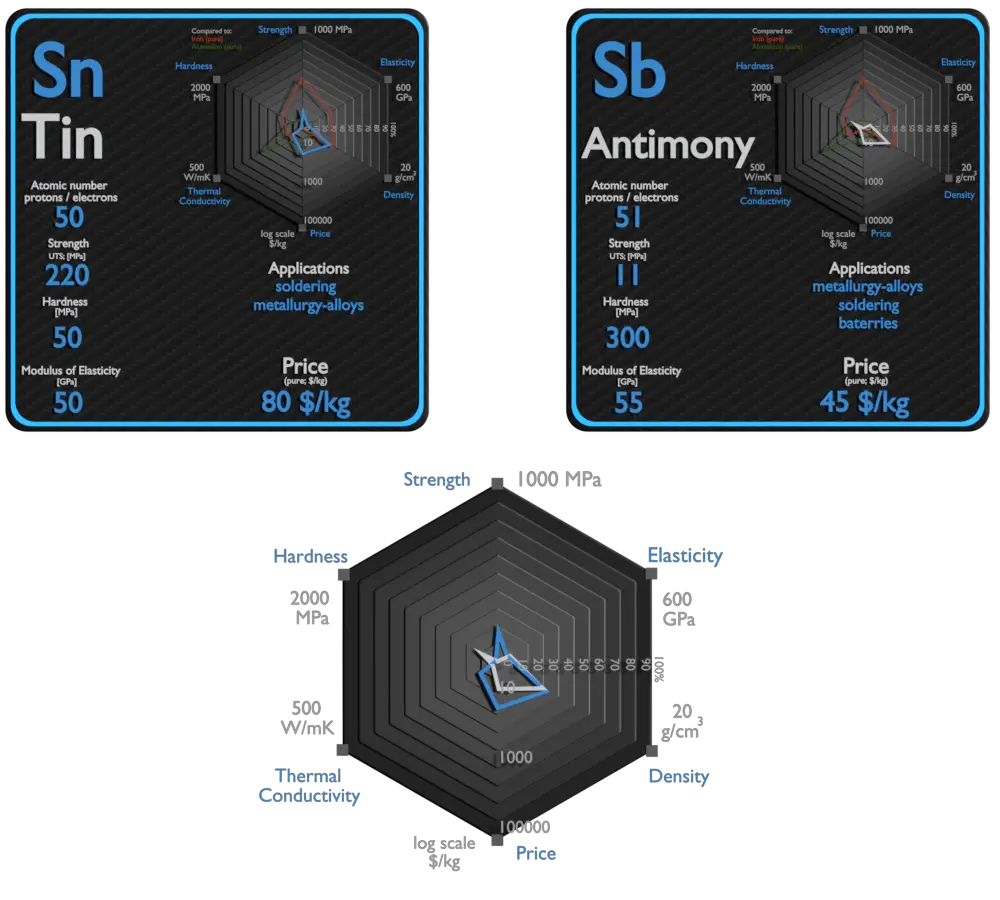
| Element | Tin | Antimony |
| Density | 7.31 g/cm3 | 6.697 g/cm3 |
| Ultimate Tensile Strength | 220 MPa | 11 MPa |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | 50 GPa | 55 GPa |
| Mohs Scale | 1.65 | 3.15 |
| Brinell Hardness | 50 MPa | 300 MPa |
| Vickers Hardness | N/A | N/A |
| Melting Point | 231.93 °C | 631 °C |
| Boiling Point | 2602 °C | 1950 °C |
| Thermal Conductivity | 67 W/mK | 24 W/mK |
| Thermal Expansion Coefficient | 22 µm/mK | 11 µm/mK |
| Specific Heat | 0.227 J/g K | 0.21 J/g K |
| Heat of Fusion | 7.029 kJ/mol | 19.87 kJ/mol |
| Heat of Vaporization | 295.8 kJ/mol | 77.14 kJ/mol |






