This article contains comparison of key thermal and atomic properties of vanadium and chromium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Vanadium vs Chromium.
Vanadium and Chromium – About Elements


Source: www.luciteria.com
Vanadium and Chromium – Applications
Vanadium
Vanadium is mainly used to produce specialty steel alloys such as high-speed tool steels, and some aluminium alloys. Vanadium is generally added to steel to inhibit grain growth during heat treatment. In controlling grain growth, it improves both the strength and toughness of hardened and tempered steels. Vanadium is added to promote abrasion resistance and to produce hard and stable carbides which being only partly soluble, release little carbon into the matrix. The most important industrial vanadium compound, vanadium pentoxide, is used as a catalyst for the production of sulfuric acid. The vanadium redox battery for energy storage may be an important application in the future.
Chromium
Chromium is one of the most important and indispensable industrial metals because of its hardness and resistance to corrosion. But it is used for more than the production of stainless steel and nonferrous alloys; it is also used to create pigments and chemicals used to process leather. In metallurgy, Chromium increases hardness, strength, and corrosion resistance. The strengthening effect of forming stable metal carbides at the grain boundaries and the strong increase in corrosion resistance made chromium an important alloying material for steel. Generally speaking, the concentration specified for most grades is approximately 4%. This level appears to result in the best balance between hardness and toughness. Chromium plays an important role in the hardening mechanism and is considered irreplaceable. At higher temperatures, chromium contributes increased strength. It is ordinarily used for applications of this nature in conjunction with molybdenum. The resistance of stainless steels is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 11% by weight, above which passivity can occur and below which it is impossible.
Vanadium and Chromium – Comparison in Table
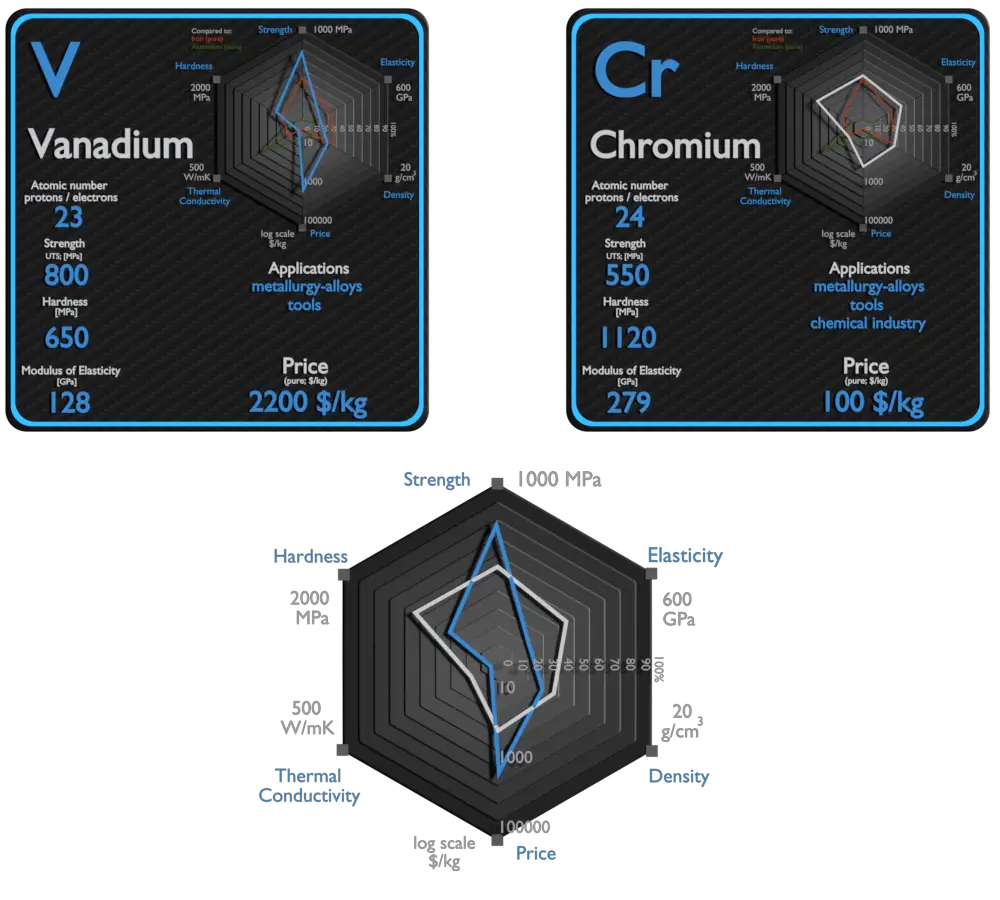
| Element | Vanadium | Chromium |
| Density | 6.11 g/cm3 | 7.14 g/cm3 |
| Ultimate Tensile Strength | 800 MPa | 550 MPa |
| Yield Strength | 770 MPa | 131 MPa |
| Young’s Modulus of Elasticity | 116 GPa | 128 GPa |
| Mohs Scale | 6 | 6.7 |
| Brinell Hardness | 700 – 2700 MPa | 650 MPa |
| Vickers Hardness | 800 – 3400 MPa | 630 MPa |
| Melting Point | 1668 °C | 1910 °C |
| Boiling Point | 3287 °C | 3407 °C |
| Thermal Conductivity | 21.9 W/mK | 30.7 W/mK |
| Thermal Expansion Coefficient | 8.6 µm/mK | 8.4 µm/mK |
| Specific Heat | 0.52 J/g K | 0.49 J/g K |
| Heat of Fusion | 15.45 kJ/mol | 20.9 kJ/mol |
| Heat of Vaporization | 421 kJ/mol | 0.452 kJ/mol |







