This article contains comparison of key thermal and atomic properties of titanium and vanadium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Titanium vs Vanadium.
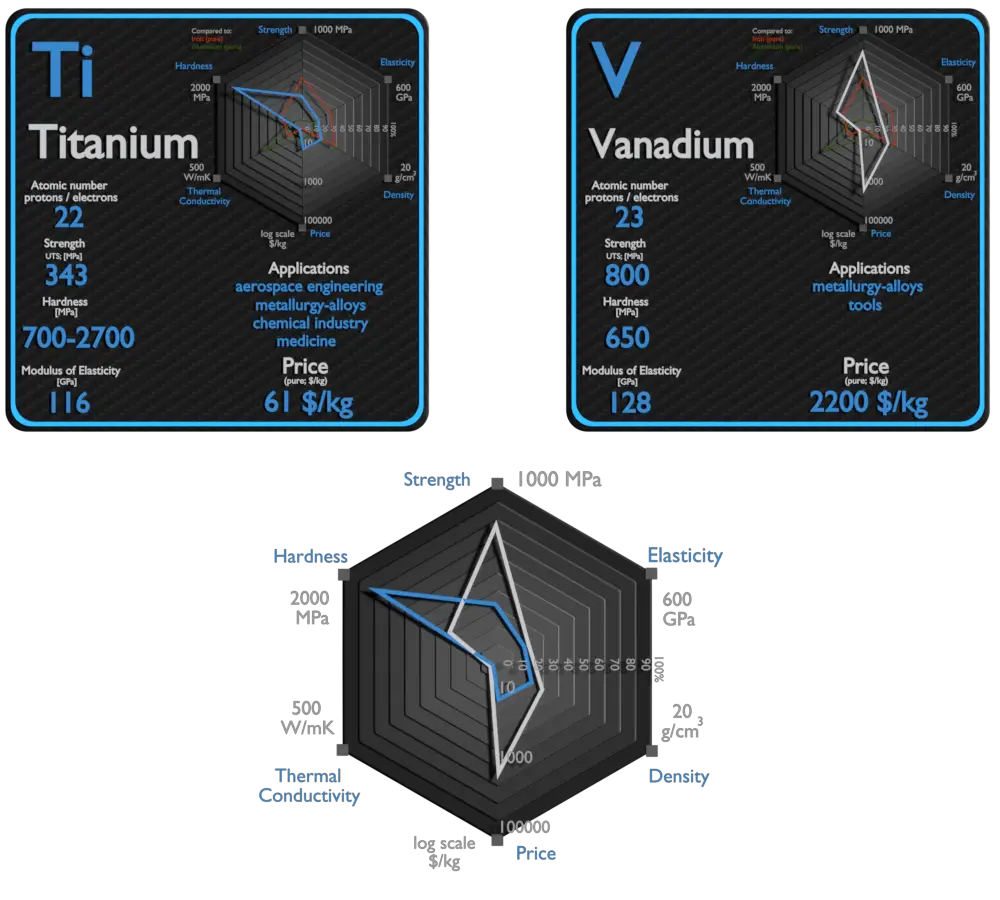
Titanium and Vanadium – About Elements


Source: www.luciteria.com
Titanium and Vanadium – Applications
Titanium
The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. These properties determine application of titanium and its alloys. The earliest production application of titanium was in 1952, for the nacelles and firewalls of the Douglas DC-7 airliner. High specific strength, good fatigue resistance and creep life, and good fracture toughness are characteristics that make titanium a preferred metal for aerospace applications. Aerospace applications, including use in both structural (airframe) components and jet engines, still account for the largest share of titanium alloy use. On the supersonic aircraft SR-71, titanium was used for 85% of the structure. Due to very high inertness, titanium has many biomedical applications, which is based on its inertness in the human body, that is, resistance to corrosion by body fluids.
Vanadium
Vanadium is mainly used to produce specialty steel alloys such as high-speed tool steels, and some aluminium alloys. Vanadium is generally added to steel to inhibit grain growth during heat treatment. In controlling grain growth, it improves both the strength and toughness of hardened and tempered steels. Vanadium is added to promote abrasion resistance and to produce hard and stable carbides which being only partly soluble, release little carbon into the matrix. The most important industrial vanadium compound, vanadium pentoxide, is used as a catalyst for the production of sulfuric acid. The vanadium redox battery for energy storage may be an important application in the future.
Titanium and Vanadium – Comparison in Table
| Element | Titanium | Vanadium |
| Density | 4.507 g/cm3 | 6.11 g/cm3 |
| Ultimate Tensile Strength | 434 MPa, 293 MPa (pure) | 800 MPa |
| Yield Strength | 380 MPa | 770 MPa |
| Young’s Modulus of Elasticity | 116 GPa | 128 GPa |
| Mohs Scale | 6 | 6.7 |
| Brinell Hardness | 700 – 2700 MPa | 650 MPa |
| Vickers Hardness | 800 – 3400 MPa | 630 MPa |
| Melting Point | 1668 °C | 1910 °C |
| Boiling Point | 3287 °C | 3407 °C |
| Thermal Conductivity | 21.9 W/mK | 30.7 W/mK |
| Thermal Expansion Coefficient | 8.6 µm/mK | 8.4 µm/mK |
| Specific Heat | 0.52 J/g K | 0.49 J/g K |
| Heat of Fusion | 15.45 kJ/mol | 20.9 kJ/mol |
| Heat of Vaporization | 421 kJ/mol | 0.452 kJ/mol |











