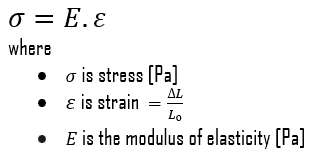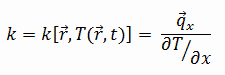Corrosion-resistant alloys, as their name indicates, are alloys with enhanced corrosion resistance. Some ferrous and many non-ferrous metals and alloys are widely used in corrosive environments. In all cases, it strongly depends on certain environment and other conditions. Corrosion-resistant alloys are used for water piping and many chemical and industrial applications. In case of ferrous alloys, we are talking about stainless steels and to some extent about cast irons. But some non-ferrous corrosion-resistant alloys exhibit remarkable corrosion resistance nad therefore they may be used for many special purposes. There are two main reasons why nonferrous materials are preferred over steels and stainless steels for many of these applications. For example, many of the non-ferrous metals and alloys possess much higher resistance to corrosion than available alloy steels and stainless steel grades. Second, a high strength-to-weight ratio or high thermal and electrical conductivity may provide a distinct advantage over a ferrous alloy.
Corrosion-resistant alloys, as their name indicates, are alloys with enhanced corrosion resistance. Some ferrous and many non-ferrous metals and alloys are widely used in corrosive environments. In all cases, it strongly depends on certain environment and other conditions. Corrosion-resistant alloys are used for water piping and many chemical and industrial applications. In case of ferrous alloys, we are talking about stainless steels and to some extent about cast irons. But some non-ferrous corrosion-resistant alloys exhibit remarkable corrosion resistance nad therefore they may be used for many special purposes. There are two main reasons why nonferrous materials are preferred over steels and stainless steels for many of these applications. For example, many of the non-ferrous metals and alloys possess much higher resistance to corrosion than available alloy steels and stainless steel grades. Second, a high strength-to-weight ratio or high thermal and electrical conductivity may provide a distinct advantage over a ferrous alloy.
Types of Corrosion-resistant Alloys
Four common non-ferrous metals used for their well-documented corrosion resistance properties are:
-

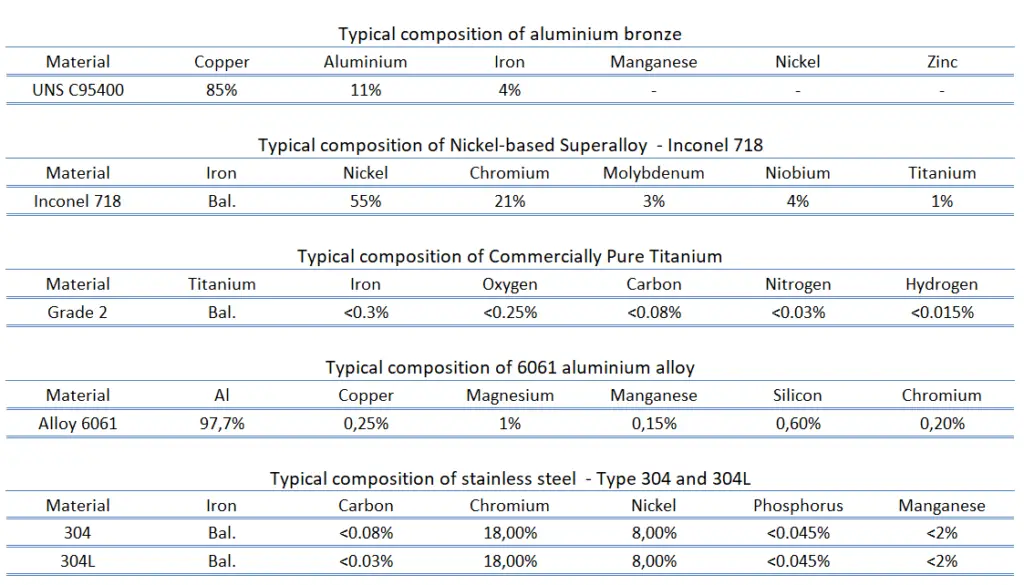
Nibral Propeller (nickel aluminium bronze) Source: generalpropeller.com Copper. Copper does not react with water, but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike the rust that forms on iron in moist air, protects the underlying metal from further corrosion (passivation). Copper nickel alloys and aluminium bronze demonstrate superior resistance to saltwater corrosion. For example, cupronickels are copper-nickel alloys that contain typically from 60 to 90 percent of copper and nickel as the main alloying element. The two main alloys are 90/10 and 70/30. Other strengthening elements, such as manganese and iron, may be also contained. Cupronickels have excellent resistance to corrosion caused by sea water. Despite its high copper content, cupronickel is silver in colour. The addition of nickel to copper also improves strength and corrosion resistance, but good ductility is retained. Cupronickels may be used in many marine applications, such as for the propellers and propeller shafts. Since cupronickel alloys have inherent resistance to macrofouling, good tensile strength, excellent ductility when annealed, high thermal conductivity and expansion characteristics, they may be used for heat exchangers, such as in steam turbine condensers, oil coolers, auxiliary cooling systems and high pressure pre-heaters at nuclear and fossil fuel power plants. Another very common corrosion-resistant material is an aluminium bronze, which has excellent corrosion resistance especially in seawater and similar environments, where the alloys often outperform many stainless steels. Their excellent resistance to corrosion results from the aluminium in the alloys, which reacts with atmospheric oxygen to form a thin, tough surface layer of alumina (aluminium oxide) which acts as a barrier to corrosion of the copper-rich alloy. They are found in wrought and cast form. Aluminium bronzes are usually golden in color. Aluminium bronzes are used in sea water applications that include:
- General sea water-related services
- Bearings
- Pipe fittings
- Pumps and valve components
- Heat exchangers
 Nickel. Nickel is a silvery-white lustrous metal with a slight golden tinge. Nickel is one of most common alloying elements. About 65% of nickel production is used in stainless steels. Because nickel does not form any carbide compounds in steel, it remains in solution in the ferrite, thus strengthening and toughening the ferrite phase. Nickel steels are easily heat treated because nickel lowers the critical cooling rate. Nickel based alloys (e.g. Fe-Cr-Ni(Mo) alloys) alloys exhibit excellent ductility and toughness, even at high strength levels and these properties are retained up to low temperatures. Nickel and its alloys are highly resistant to corrosion in many environments, especially those that are basic (alkaline). Nickel also reduces thermal expansion for better dimensional stability. Nickel is the base elements for superalloys. These metals have excellent resistance to thermal creep deformation and retain their stiffness, strength, toughness and dimensional stability at temperatures much higher than the other aerospace structural materials. For example, Inconel is a registered trademark of Special Metals for a family of austenitic nickel-chromium-based superalloys. Inconel 718 is a nickel-based superalloy that possesses high strength properties and resistance to elevated temperatures. It also demonstrates remarkable protection against corrosion and oxidation. They were initially developed for use in aircraft piston engine turbosuperchargers. Today, the most common application is in aircraft turbine components, which must withstand exposure to severely oxidizing environments and high temperatures for reasonable time periods.
Nickel. Nickel is a silvery-white lustrous metal with a slight golden tinge. Nickel is one of most common alloying elements. About 65% of nickel production is used in stainless steels. Because nickel does not form any carbide compounds in steel, it remains in solution in the ferrite, thus strengthening and toughening the ferrite phase. Nickel steels are easily heat treated because nickel lowers the critical cooling rate. Nickel based alloys (e.g. Fe-Cr-Ni(Mo) alloys) alloys exhibit excellent ductility and toughness, even at high strength levels and these properties are retained up to low temperatures. Nickel and its alloys are highly resistant to corrosion in many environments, especially those that are basic (alkaline). Nickel also reduces thermal expansion for better dimensional stability. Nickel is the base elements for superalloys. These metals have excellent resistance to thermal creep deformation and retain their stiffness, strength, toughness and dimensional stability at temperatures much higher than the other aerospace structural materials. For example, Inconel is a registered trademark of Special Metals for a family of austenitic nickel-chromium-based superalloys. Inconel 718 is a nickel-based superalloy that possesses high strength properties and resistance to elevated temperatures. It also demonstrates remarkable protection against corrosion and oxidation. They were initially developed for use in aircraft piston engine turbosuperchargers. Today, the most common application is in aircraft turbine components, which must withstand exposure to severely oxidizing environments and high temperatures for reasonable time periods.- Titanium. Pure titanium is stronger than common, low-carbon steels, but 45% lighter. It is also twice as strong as weak aluminium alloys but only 60% heavier. The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. Titanium’s corrosion resistance is based on the formation of a stable, protective oxide layer. Although “commercially pure” titanium has acceptable mechanical properties and has been used for orthopedic and dental implants, for most applications titanium is alloyed with small amounts of aluminium and vanadium, typically 6% and 4% respectively, by weight. This mixture has a solid solubility which varies dramatically with temperature, allowing it to undergo precipitation strengthening. Titanium alloys are metals that contain a mixture of titanium and other chemical elements. Such alloys have very high tensile strength and toughness (even at extreme temperatures). They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures. For example, commercially pure titanium grade 2 is very similar to grade 1, but it has higher strength than grade 1 and excellent cold forming properties. It provides excellent welding properties and has excellent resistance to oxidation and corrosion. This grade of titanium is the most common grade of the commercially pure titanium industry. It is the prime choice for many fields of applications:
- Aerospace,
- Automotive,
- Chemical Processing & Chlorate Manufacturing,
- Desalination
- Power generation
- Aluminium. In general, aluminium alloys are characterized by a relatively low density (2.7 g/cm3 as compared to 7.9 g/cm3 for steel), high electrical and thermal conductivities, and a resistance to corrosion in some common environments, including the ambient atmosphere. Its corrosion resitance strongly depends on certain alloy. For example, duralumin is due to presence of copper susceptible to corrosion, while 6061 alloy remains resistant to corrosion even when the surface is abraded. At higher temperature and in aggressive environments, aluminium alloys have poor corrosion resistance and they also suffer from stress corrosion cracking.
Stainless Steels – Ferrous Corrosion-resistant Alloys
Although we are discussing primarily non-ferrous corrosion resistant alloys, we have to mention stainless steels. Stainless steels are defined as low-carbon steels with at least 10.5% chromium with or without other alloying elements and a maximum of 1.2% carbon by mass. Stainless steels, also known as inox steels or inox from French inoxydable (inoxidizable), are steel alloys, which are very well known for their corrosion resistance, which increases with increasing chromium content. Corrosion resistance may also be enhanced by nickel and molybdenum additions.
 Austenitic stainless steels have the best corrosion resistance of all stainless steels and they have excellent cryogenic properties, and good high-temperature strength. They possess a face-centered cubic (fcc) microstructure that is nonmagnetic, and they can be easily welded. This austenite crystalline structure is achieved by sufficient additions of the austenite stabilizing elements nickel, manganese and nitrogen. Austenitic stainless steel is the largest family of stainless steels, making up about two-thirds of all stainless steel production.
Austenitic stainless steels have the best corrosion resistance of all stainless steels and they have excellent cryogenic properties, and good high-temperature strength. They possess a face-centered cubic (fcc) microstructure that is nonmagnetic, and they can be easily welded. This austenite crystalline structure is achieved by sufficient additions of the austenite stabilizing elements nickel, manganese and nitrogen. Austenitic stainless steel is the largest family of stainless steels, making up about two-thirds of all stainless steel production.
The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below which it is impossible. Strength and corrosion resistance of stainless steel often make it the material of choice in transportation and processing equipment, engine parts, and firearms. Most of the structural applications occur in the chemical and power engineering industries, which account for more than third of the market for stainless steel products. The wide variety of applications includes nuclear reactor vessels, heat exchangers.
Stress-corrosion cracking
One of the most serious metallurgical problems and one that is a major concern in the nuclear industry is stress-corrosion cracking (SCC). Stress-corrosion cracking results from the combined action of an applied tensile stress and a corrosive environment, both influences are necessary. SCC is a type of intergranular attack corrosion that occurs at the grain boundaries under tensile stress. Low alloy steels are less susceptible than high alloy steels, but they are subject to SCC in water containing chloride ions. Nickel-based alloys, however, are not effected by chloride or hydroxide ions. An example of a nickel-based alloy that is resistant to stress-corrosion cracking is Inconel.
Properties of Corrosion-resistant Alloys
Material properties are intensive properties, that means they are independent of the amount of mass and may vary from place to place within the system at any moment. The basis of materials science involves studying the structure of materials, and relating them to their properties (mechanical, electrical etc.). Once a materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and the way in which it has been processed into its final form.
Density of Corrosion-resistant Alloys
Density of typical aluminium bronze is 7.45 g/cm3 (UNS C95400).
Density of typical superalloy is 8.22 g/cm3 (Inconel 718).
Density of typical titanium alloy is 4.51 g/cm3 (Grade 2).
Density of typical aluminium alloy is 2.7 g/cm3 (6061 alloy).
Density of typical stainless steel is 8.0 g/cm3 (304 steel).
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
In words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/m3). The Standard English unit is pounds mass per cubic foot (lbm/ft3).
Since the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance, it is obvious, the density of a substance strongly depends on its atomic mass and also on the atomic number density (N; atoms/cm3),
- Atomic Weight. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Therefore it is determined by the mass number (number of protons and neutrons).
- Atomic Number Density. The atomic number density (N; atoms/cm3), which is associated with atomic radii, is the number of atoms of a given type per unit volume (V; cm3) of the material. The atomic number density (N; atoms/cm3) of a pure material having atomic or molecular weight (M; grams/mol) and the material density (⍴; gram/cm3) is easily computed from the following equation using Avogadro’s number (NA = 6.022×1023 atoms or molecules per mole):
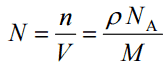
- Crystal Structure. Density of crystalline substance is significantly affected by its crystal structure. FCC structure, along with its hexagonal relative (hcp), has the most efficient packing factor (74%). Metals containing FCC structures include austenite, aluminum, copper, lead, silver, gold, nickel, platinum, and thorium.
Mechanical Properties of Corrosion-resistant Alloys
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial and engineers must take them into account.
Strength of Corrosion-resistant Alloys
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. Strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
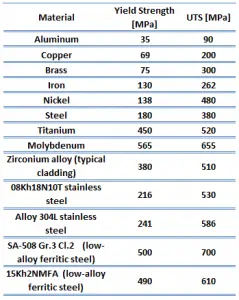
Ultimate tensile strength of aluminium bronze – UNS C95400 is about 550 MPa.
Ultimate tensile strength of superalloy – Inconel 718 depends on heat treatment process, but it is about 1200 MPa.
Ultimate tensile strength of commercially pure titanium – Grade 2 is about 340 MPa.
Ultimate tensile strength of 6061 aluminium alloy depends greatly on the temper of the material, but for T6 temper it is about 290 MPa.
Ultimate tensile strength of stainless steel – type 304 is 515 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
Yield Strength
Yield strength of aluminium bronze – UNS C95400 is about 250 MPa.
Yield strength of superalloy – Inconel 718 depends on heat treatment process, but it is about 1030 MPa.
Yield strength of commercially pure titanium – Grade 2 is about 300 MPa.
Yield strength of 6061 aluminium alloy depends greatly on the temper of the material, but for T6 temper it is about 240 MPa.
Yield strength of stainless steel – type 304 is 205 MPa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins. Prior to the yield point, the material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behaviour termed a yield point phenomenon. Yield strengths vary from 35 MPa for a low-strength aluminum to greater than 1400 MPa for very high-strength steels.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of aluminium bronze – UNS C95400 is about 110 GPa.
Young’s modulus of elasticity of superalloy – Inconel 718 is 200 GPa.
Young’s modulus of elasticity of commercially pure titanium – Grade 2 is about 105 GPa.
Young’s modulus of elasticity of 6061 aluminium alloy is about 69 GPa.
Young’s modulus of elasticity stainless steel – type 304 and 304L is 193 GPa.
The Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to a limiting stress, a body will be able to recover its dimensions on removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position. All the atoms are displaced the same amount and still maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions and no permanent deformation occurs. According to the Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
Hardness of Corrosion-resistant Alloys
Brinell hardness of aluminium bronze – UNS C95400 is approximately 170 MPa.
Brinell hardness of superalloy – Inconel 718 depends on heat treatment process, but it is approximately 330 MPa.
Rockwell hardness of commercially pure titanium – Grade 2 is approximately 80 HRB.
Brinell hardness of 6061 aluminium alloy depends greatly on the temper of the material, but for T6 temper it is approximately 95 MPa.
Brinell hardness of stainless steel – type 304 is approximately 201 MPa.
Rockwell hardness test is one of the most common indentation hardness tests, that has been developed for hardness testing. In contrast to Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position. The major load is applied, then removed while still maintaining the minor load. The difference between depth of penetration before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Corrosion-resistant Alloys
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Corrosion-resistant Alloys
Melting point of aluminium bronze – UNS C95400 is around 1030°C.
Melting point of superalloy – Inconel 718 steel is around 1400°C.
Melting point of commercially pure titanium – Grade 2 is around 1660°C.
Melting point of 6061 aluminium alloy is around 600°C.
Melting point of stainless steel – type 304 steel is around 1450°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Corrosion-resistant Alloys
The thermal conductivity of aluminium bronze – UNS C95400 is 59 W/(m.K).
The thermal conductivity of superalloy – Inconel 718 is 6.5 W/(m.K).
The thermal conductivity of commercially pure titanium – Grade 2 is 16 W/(m.K).
The thermal conductivity of 6061 aluminium alloy is 150 W/(m.K).
The thermal conductivity of stainless steel – type 304 is 20 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Corrosion-resistant Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.