This article contains comparison of key thermal and atomic properties of hydrogen and fluorine, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Hydrogen vs Fluorine.
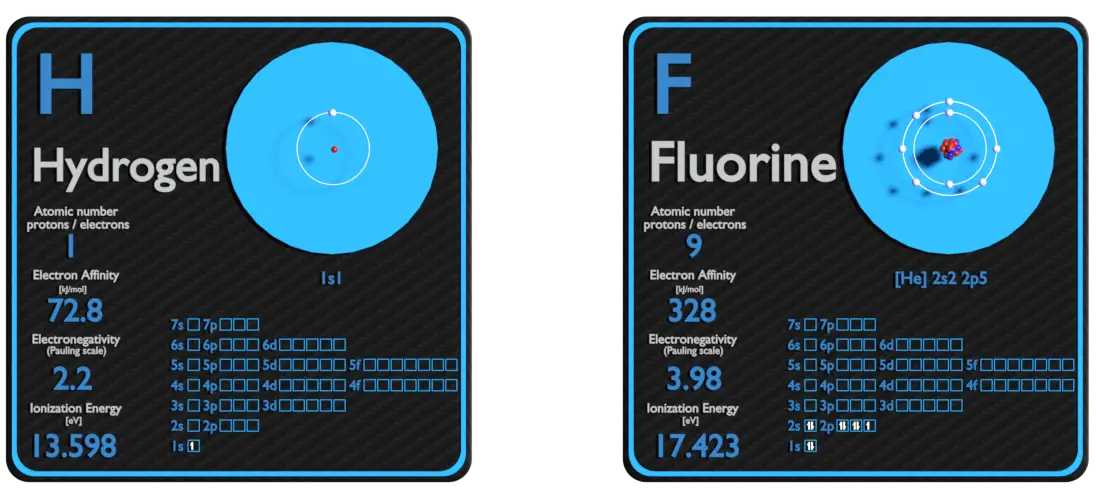
Hydrogen and Fluorine – About Elements


Source: www.luciteria.com
Hydrogen and Fluorine – Applications
Hydrogen
Hydrogen is versatile and can be utilized in various ways. These multiple uses can be grouped into two large categories. Hydrogen as a feedstock. A role whose importance is being recognized for decades and will continue to grow and evolve. The largest single use of hydrogen in the world is in ammonia manufacture, which consumes about two-thirds of the world’s hydrogen production. Hydrogen is versatile and can be utilized in various ways. These multiple uses can be grouped into two large categories. Hydrogen as a feedstock for further chemical processes. A role whose importance is being recognized for decades and will continue to grow and evolve. And hydrogen as an energy carrier. Hydrogen is also commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules.
Fluorine
Owing to the expense of refining pure fluorine, most commercial applications use fluorine compounds, with about half of mined fluorite used in steelmaking. The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides, or into cryolite, which plays a key role in aluminium refining. Most commercial uranium enrichment processes (gaseous diffusion and the gas centrifuge method) require the uranium to be in a gaseous form, therefore the uranium oxide concentrate must be first converted to uranium hexafluoride, which is a gas at relatively low temperatures. Molecules containing a carbon–fluorine bond often have very high chemical and thermal stability; their major uses are as refrigerants, electrical insulation and cookware, the last as PTFE (Teflon).
Hydrogen and Fluorine – Comparison in Table
| Element | Hydrogen | Fluorine |
| Density | 0.00009 g/cm3 | 0.0017 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -259.1 °C | -219.8 °C |
| Boiling Point | -252.9 °C | -188.1 °C |
| Thermal Conductivity | 0.1805 W/mK | 0.0279 W/mK |
| Thermal Expansion Coefficient | — µm/mK | — µm/mK |
| Specific Heat | 14.304 J/g K | 0.82 J/g K |
| Heat of Fusion | 0.05868 kJ/mol | 0.2552 kJ/mol |
| Heat of Vaporization | 0.44936 kJ/mol | 3.2698 kJ/mol |