This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Fluorine vs Chlorine.
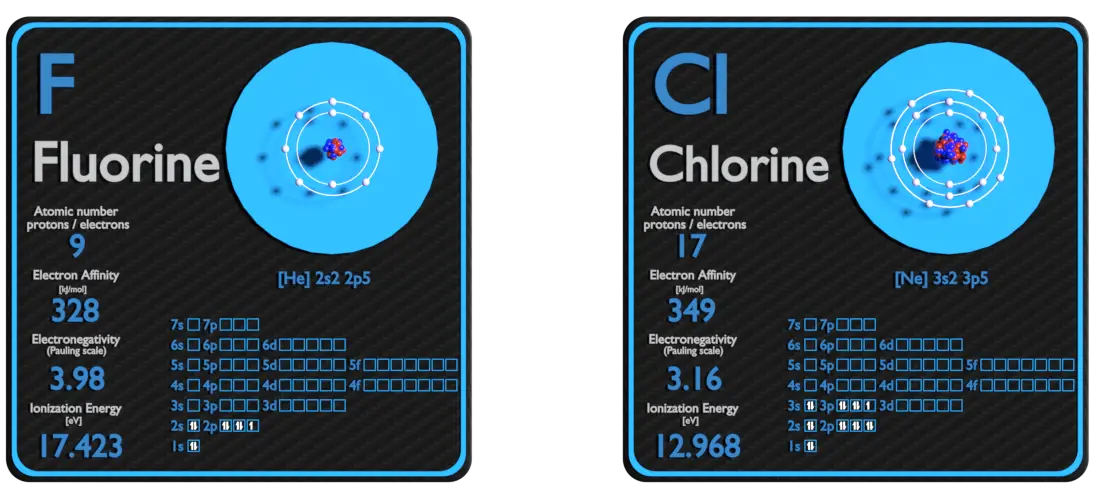
Fluorine and Chlorine – About Elements


Source: www.luciteria.com
Fluorine and Chlorine – Applications
Fluorine
Owing to the expense of refining pure fluorine, most commercial applications use fluorine compounds, with about half of mined fluorite used in steelmaking. The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides, or into cryolite, which plays a key role in aluminium refining. Most commercial uranium enrichment processes (gaseous diffusion and the gas centrifuge method) require the uranium to be in a gaseous form, therefore the uranium oxide concentrate must be first converted to uranium hexafluoride, which is a gas at relatively low temperatures. Molecules containing a carbon–fluorine bond often have very high chemical and thermal stability; their major uses are as refrigerants, electrical insulation and cookware, the last as PTFE (Teflon).
Chlorine
Chlorine is used in the manufacture of a wide range of consumer products, about two-thirds of them organic chemicals such as polyvinyl chloride (PVC), many intermediates for the production of plastics, and other end products which do not contain the element. As a common disinfectant, elemental chlorine and chlorine-generating compounds are used more directly in swimming pools to keep them sanitary. While perhaps best known for its role in providing clean drinking water, chlorine chemistry also helps provide energy-efficient building materials, electronics, fiber optics, solar energy cells, 93 percent of life-saving pharmaceuticals, 86 percent of crop protection compounds, medical plastics, and much more.
Fluorine and Chlorine – Comparison in Table
| Element | Fluorine | Chlorine |
| Density | 0.0017 g/cm3 | 0.0032 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -219.8 °C | -101 °C |
| Boiling Point | -188.1 °C | -34.6 °C |
| Thermal Conductivity | 0.0279 W/mK | 0.0089 W/mK |
| Thermal Expansion Coefficient | N/A | N/A |
| Specific Heat | 0.82 J/g K | 0.48 J/g K |
| Heat of Fusion | 0.2552 kJ/mol | 3.23 kJ/mol |
| Heat of Vaporization | 3.2698 kJ/mol | 10.2 kJ/mol |


















