This article contains comparison of key thermal and atomic properties of oxygen and fluorine, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Oxygen vs Fluorine.
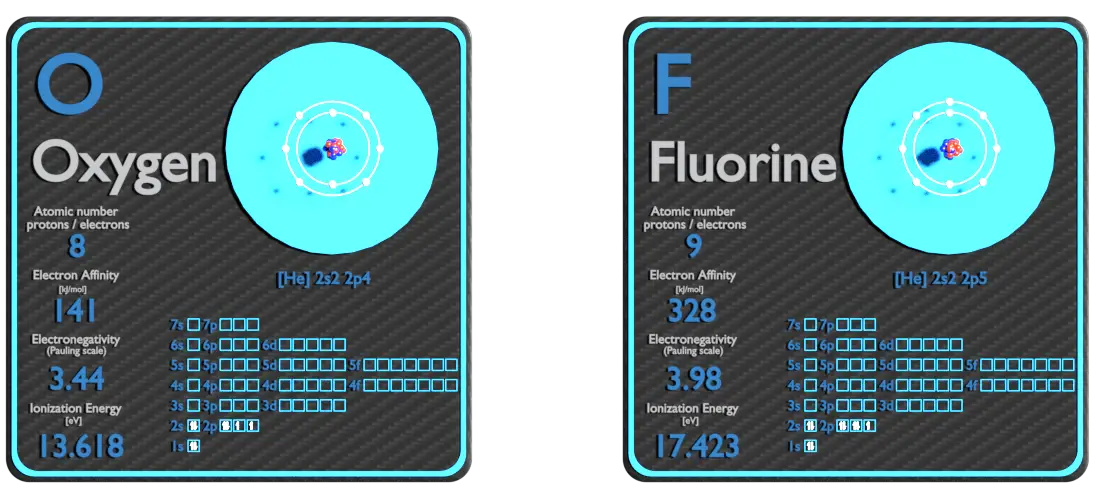
Oxygen and Fluorine – About Elements


Source: www.luciteria.com
Oxygen and Fluorine – Applications
Oxygen
Common uses of oxygen include production of steel, plastics and textiles, brazing, welding and cutting of steels and other metals, rocket propellant, oxygen therapy, and life support systems in aircraft, submarines, spaceflight and diving. Smelting of iron ore into steel consumes 55% of commercially produced oxygen. In this process, oxygen is injected through a high-pressure lance into molten iron, which removes sulfur impurities and excess carbon as the respective oxides, sulfur dioxide and carbon dioxide. Uptake of oxygen from the air is the essential purpose of respiration, so oxygen supplementation is used in medicine. Treatment not only increases oxygen levels in the patient’s blood, but has the secondary effect of decreasing resistance to blood flow in many types of diseased lungs, easing work load on the heart.
Fluorine
Owing to the expense of refining pure fluorine, most commercial applications use fluorine compounds, with about half of mined fluorite used in steelmaking. The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides, or into cryolite, which plays a key role in aluminium refining. Most commercial uranium enrichment processes (gaseous diffusion and the gas centrifuge method) require the uranium to be in a gaseous form, therefore the uranium oxide concentrate must be first converted to uranium hexafluoride, which is a gas at relatively low temperatures. Molecules containing a carbon–fluorine bond often have very high chemical and thermal stability; their major uses are as refrigerants, electrical insulation and cookware, the last as PTFE (Teflon).
Oxygen and Fluorine – Comparison in Table
| Element | Oxygen | Fluorine |
| Density | 0.00125 g/cm3 | 0.0017 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -209.9 °C | -219.8 °C |
| Boiling Point | -195.8 °C | -188.1 °C |
| Thermal Conductivity | 0.02598 W/mK | 0.0279 W/mK |
| Thermal Expansion Coefficient | N/A | N/A |
| Specific Heat | 1.04 J/g K | 0.82 J/g K |
| Heat of Fusion | (N2) 0.7204 kJ/mol | 0.2552 kJ/mol |
| Heat of Vaporization | (N2) 5.56 kJ/mol | 3.2698 kJ/mol |