This article contains comparison of key thermal and atomic properties of hydrogen and oxygen, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Hydrogen vs Oxygen.
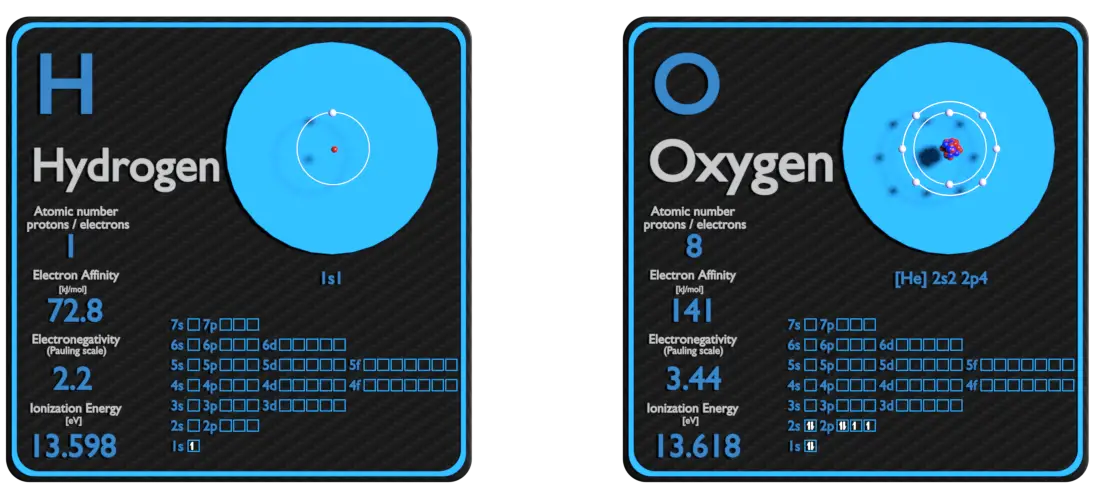
Hydrogen and Oxygen – About Elements


Source: www.luciteria.com
Hydrogen and Oxygen – Applications
Hydrogen
Hydrogen is versatile and can be utilized in various ways. These multiple uses can be grouped into two large categories. Hydrogen as a feedstock. A role whose importance is being recognized for decades and will continue to grow and evolve. The largest single use of hydrogen in the world is in ammonia manufacture, which consumes about two-thirds of the world’s hydrogen production. Hydrogen is versatile and can be utilized in various ways. These multiple uses can be grouped into two large categories. Hydrogen as a feedstock for further chemical processes. A role whose importance is being recognized for decades and will continue to grow and evolve. And hydrogen as an energy carrier. Hydrogen is also commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules.
Oxygen
Common uses of oxygen include production of steel, plastics and textiles, brazing, welding and cutting of steels and other metals, rocket propellant, oxygen therapy, and life support systems in aircraft, submarines, spaceflight and diving. Smelting of iron ore into steel consumes 55% of commercially produced oxygen. In this process, oxygen is injected through a high-pressure lance into molten iron, which removes sulfur impurities and excess carbon as the respective oxides, sulfur dioxide and carbon dioxide. Uptake of oxygen from the air is the essential purpose of respiration, so oxygen supplementation is used in medicine. Treatment not only increases oxygen levels in the patient’s blood, but has the secondary effect of decreasing resistance to blood flow in many types of diseased lungs, easing work load on the heart.
Hydrogen and Oxygen – Comparison in Table
| Element | Hydrogen | Oxygen |
| Density | 0.00009 g/cm3 | 0.00143 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -259.1 °C | -218.4 °C |
| Boiling Point | -252.9 °C | -183 °C |
| Thermal Conductivity | 0.1805 W/mK | 0.02674 W/mK |
| Thermal Expansion Coefficient | — µm/mK | — µm/mK |
| Specific Heat | 14.304 J/g K | 0.92 J/g K |
| Heat of Fusion | 0.05868 kJ/mol | (O2) 0.444 kJ/mol |
| Heat of Vaporization | 0.44936 kJ/mol | (O2) 6.82 kJ/mol |