This article contains comparison of key thermal and atomic properties of rhodium and gold, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Rhodium vs Gold.
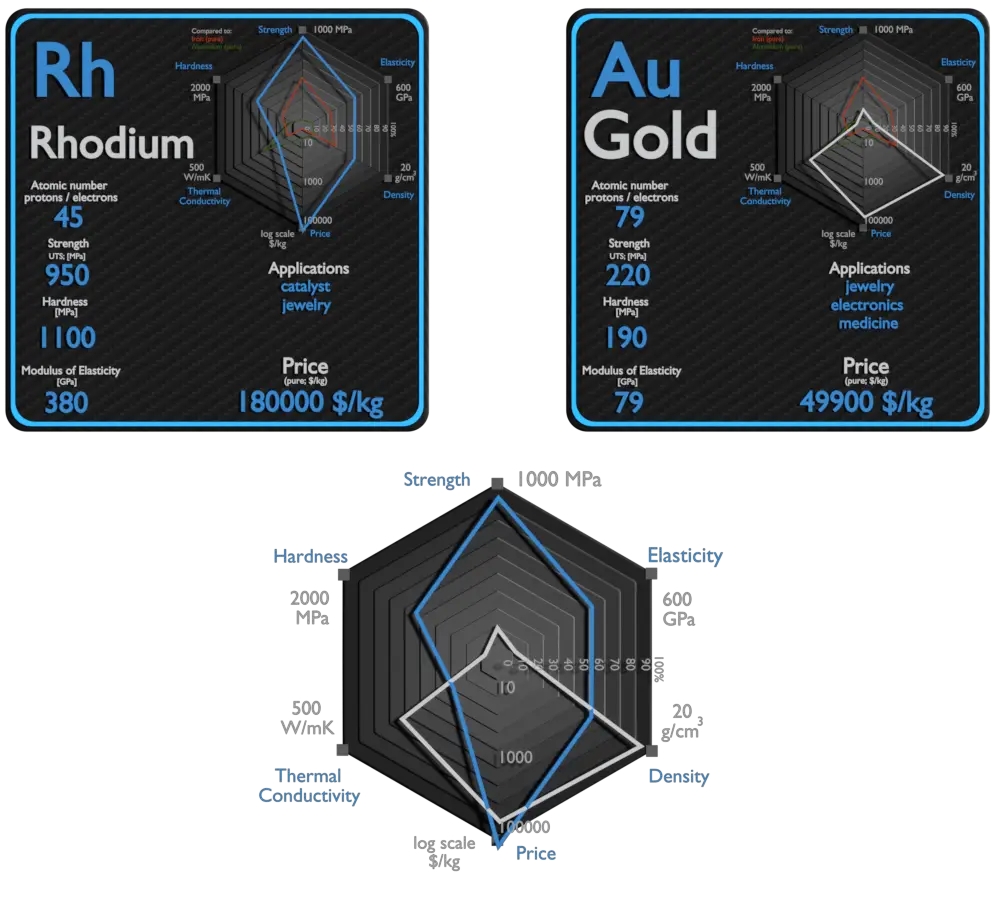
Rhodium and Gold – About Elements


Source: www.luciteria.com
Rhodium and Gold – Applications
Rhodium
The element’s major use (approximately 80% of world rhodium production) is as one of the catalysts in the three-way catalytic converters in automobiles. Because rhodium metal is inert against corrosion and most aggressive chemicals, and because of its rarity, rhodium is usually alloyed with platinum or palladium and applied in high-temperature and corrosion-resistive coatings. In nuclear reactors, rhodium-based detectors are often used for incore neutron flux measuring.
Gold
Gold is used extensively in jewellery, either in its pure form or as an alloy. About 75% of all gold produced is used in the jewelry industry. Pure gold is too soft to stand up to the stresses applied to many jewelry items. Craftsmen learned that alloying gold with other metals such as copper, silver, and platinum would increase its durability. The term ‘carat’ indicates the amount of gold present in an alloy. 24-carat is pure gold, but it is very soft. 18- and 9-carat gold alloys are commonly used because they are more durable. Gold’s high malleability, ductility, resistance to corrosion and most other chemical reactions, and conductivity of electricity have led to its continued use in corrosion resistant electrical connectors in all types of computerized devices (its chief industrial use). Gold is also used in infrared shielding, colored-glass production, gold leafing, and tooth restoration. Only 10% of the world consumption of new gold produced goes to industry, but by far the most important industrial use for new gold is in fabrication of corrosion-free electrical connectors in computers and other electrical devices.
Rhodium and Gold – Comparison in Table
| Element | Rhodium | Gold |
| Density | 12.45 g/cm3 | 19.3 g/cm3 |
| Ultimate Tensile Strength | 950 MPa | 220 MPa |
| Yield Strength | N/A | 205 MPa |
| Young’s Modulus of Elasticity | 380 GPa | 79 GPa |
| Mohs Scale | 6 | 2.75 |
| Brinell Hardness | 1100 MPa | 190 MPa |
| Vickers Hardness | 1246 MPa | 215 MPa |
| Melting Point | 1964 °C | 1064 °C |
| Boiling Point | 3695 °C | 2970 °C |
| Thermal Conductivity | 150 W/mK | 320 W/mK |
| Thermal Expansion Coefficient | 8.2 µm/mK | 14.2 µm/mK |
| Specific Heat | 0.242 J/g K | 0.128 J/g K |
| Heat of Fusion | 21.5 kJ/mol | 12.55 kJ/mol |
| Heat of Vaporization | 493 kJ/mol | 334.4 kJ/mol |