This article contains comparison of key thermal and atomic properties of rhodium and silver, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Rhodium vs Silver.
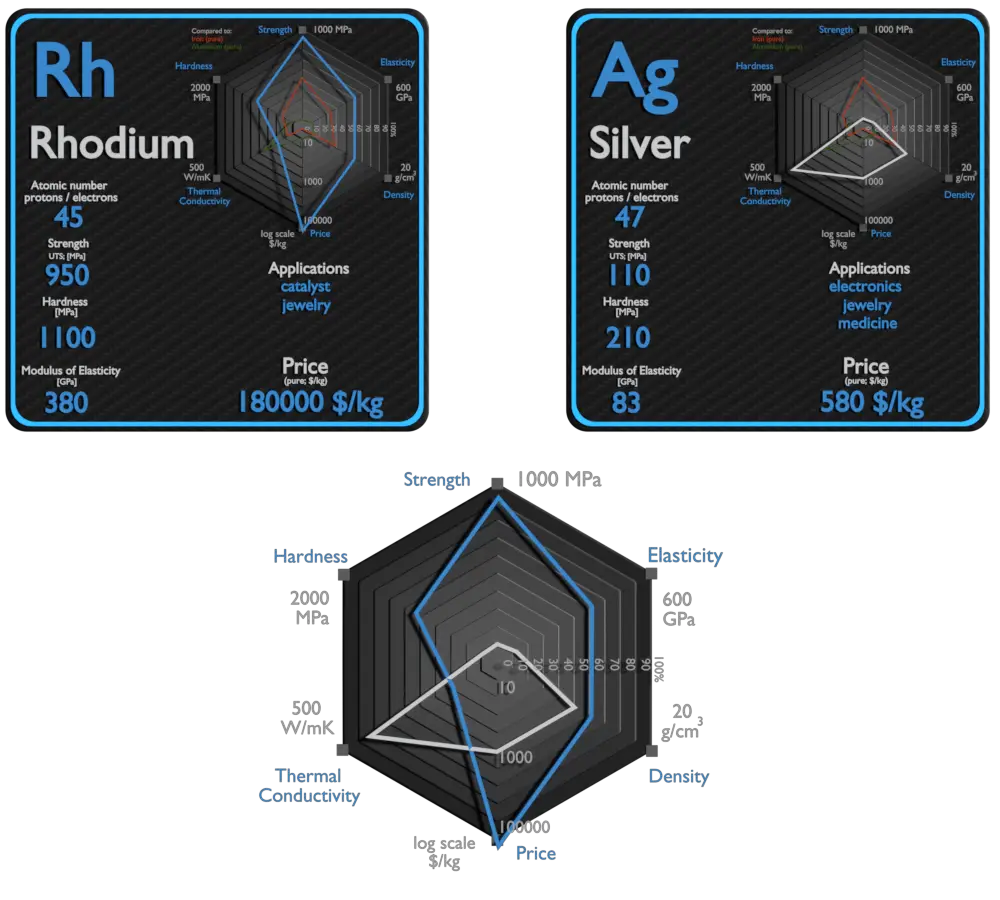
Rhodium and Silver – About Elements


Source: www.luciteria.com
Rhodium and Silver – Applications
Rhodium
The element’s major use (approximately 80% of world rhodium production) is as one of the catalysts in the three-way catalytic converters in automobiles. Because rhodium metal is inert against corrosion and most aggressive chemicals, and because of its rarity, rhodium is usually alloyed with platinum or palladium and applied in high-temperature and corrosion-resistive coatings. In nuclear reactors, rhodium-based detectors are often used for incore neutron flux measuring.
Silver
Silver has long been valued as a precious metal. Silver metal is used in many bullion coins, sometimes alongside gold. Silver has many important, far-reaching technological and electronic applications. It’s used in everything from cell phones, computers and semiconductors to automobiles, water-purification systems and—because it is the best conductor of heat of all elements—spacecraft solar radiation tiles. Silver is of the upmost importance in photography (where approximately 30% of the U.S. Industrial consumption goes into this application). The medical uses of silver include its use in wound dressings, creams, and as an antibiotic coating on medical devices. Wound dressings containing silver sulfadiazine or silver nanomaterials may be used on external infections.
Rhodium and Silver – Comparison in Table
| Element | Rhodium | Silver |
| Density | 12.45 g/cm3 | 10.49 g/cm3 |
| Ultimate Tensile Strength | 950 MPa | 110 MPa |
| Yield Strength | N/A | 45 MPa |
| Young’s Modulus of Elasticity | 380 GPa | 83 GPa |
| Mohs Scale | 6 | 3.25 |
| Brinell Hardness | 1100 MPa | 210 MPa |
| Vickers Hardness | 1246 MPa | 251 MPa |
| Melting Point | 1964 °C | 961.78 °C |
| Boiling Point | 3695 °C | 2162 °C |
| Thermal Conductivity | 150 W/mK | 430 W/mK |
| Thermal Expansion Coefficient | 8.2 µm/mK | 18.9 µm/mK |
| Specific Heat | 0.242 J/g K | 0.235 J/g K |
| Heat of Fusion | 21.5 kJ/mol | 11.3 kJ/mol |
| Heat of Vaporization | 493 kJ/mol | 250.58 kJ/mol |










