This article contains comparison of key thermal and atomic properties of titanium and zinc, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Titanium vs Zinc.
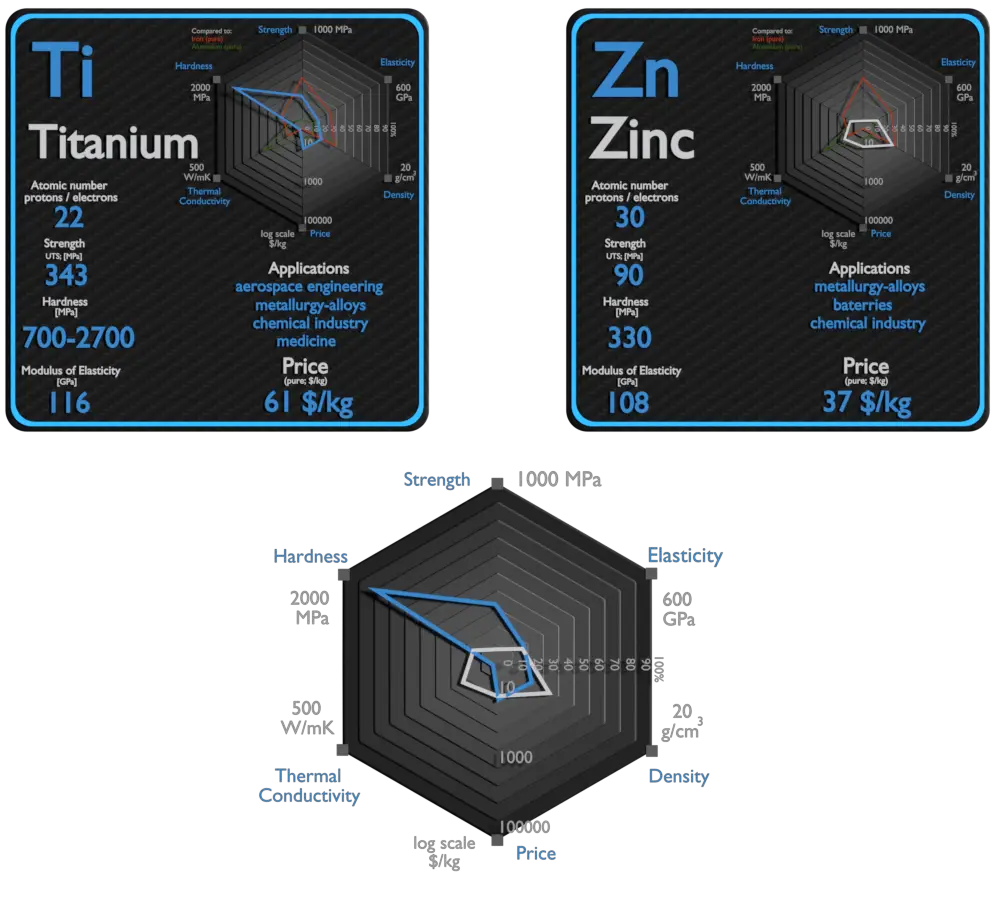
Titanium and Zinc – About Elements


Source: www.luciteria.com
Titanium and Zinc – Applications
Titanium
The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. These properties determine application of titanium and its alloys. The earliest production application of titanium was in 1952, for the nacelles and firewalls of the Douglas DC-7 airliner. High specific strength, good fatigue resistance and creep life, and good fracture toughness are characteristics that make titanium a preferred metal for aerospace applications. Aerospace applications, including use in both structural (airframe) components and jet engines, still account for the largest share of titanium alloy use. On the supersonic aircraft SR-71, titanium was used for 85% of the structure. Due to very high inertness, titanium has many biomedical applications, which is based on its inertness in the human body, that is, resistance to corrosion by body fluids.
Zinc
Corrosion-resistant zinc plating of iron (hot-dip galvanizing) is the major application for zinc. Coating of steel constitutes the largest single use of zinc, but it is used in large tonnages in zinc alloy castings, as zinc dust and oxide, and in wrought zinc products. Galvanized steel is just plain carbon steel that has been coated with a thin zinc layer. The zinc protects iron by corroding first, but zinc corrodes at much lower rates than do steel. Other applications are in electrical batteries, small non-structural castings, and alloys such as brass. A variety of zinc compounds are commonly used, such as zinc carbonate and zinc gluconate (as dietary supplements), zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and dimethylzinc or diethylzinc in the organic laboratory. A key part of the modern materials world in which zinc finds itself is recycling. Zinc, in common with all metals (and unlike synthetic materials) can be recycled indefinitely without degradation.
Titanium and Zinc – Comparison in Table
| Element | Titanium | Zinc |
| Density | 4.507 g/cm3 | 7.14 g/cm3 |
| Ultimate Tensile Strength | 434 MPa, 293 MPa (pure) | 90 MPa |
| Yield Strength | 380 MPa | 75 MPa |
| Young’s Modulus of Elasticity | 116 GPa | 108 GPa |
| Mohs Scale | 6 | 2.5 |
| Brinell Hardness | 700 – 2700 MPa | 330 MPa |
| Vickers Hardness | 800 – 3400 MPa | N/A |
| Melting Point | 1668 °C | 419.53 °C |
| Boiling Point | 3287 °C | 907 °C |
| Thermal Conductivity | 21.9 W/mK | 116 W/mK |
| Thermal Expansion Coefficient | 8.6 µm/mK | 30.2 µm/mK |
| Specific Heat | 0.52 J/g K | 0.39 J/g K |
| Heat of Fusion | 15.45 kJ/mol | 7.322 kJ/mol |
| Heat of Vaporization | 421 kJ/mol | 115.3 kJ/mol |



















