This article contains comparison of key thermal and atomic properties of beryllium and chlorine, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Beryllium vs Chlorine.
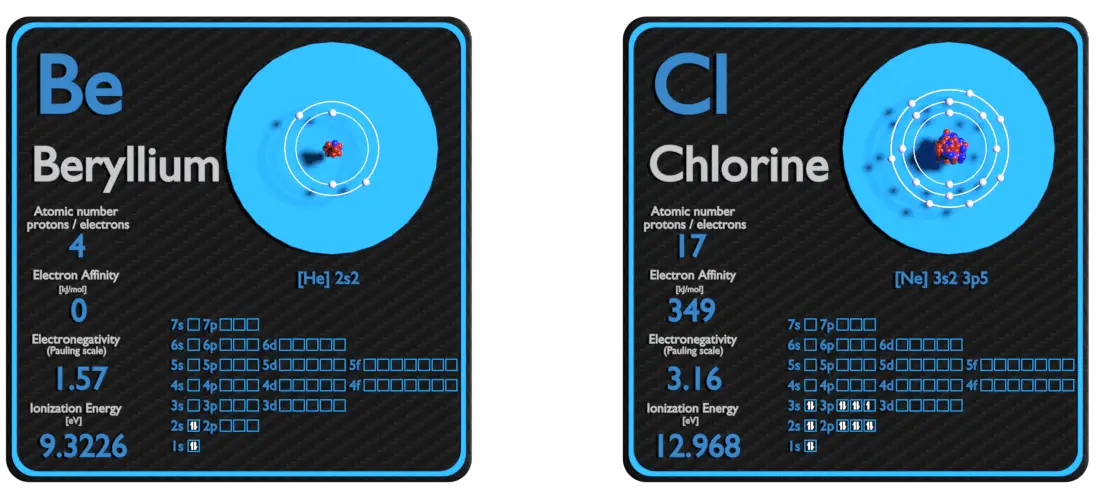
Beryllium and Chlorine – About Elements


Source: www.luciteria.com
Beryllium and Chlorine – Applications
Beryllium
Berylium can be utilized as alloying agent in production of beryllium-copper, X-ray detection diagnostics, manufacture of computer peripherals, in nuclear reactors as neutron moderators and reflectors. 80% of the beryllium used goes into copper beryllium alloys. The combination of light weight with high strength at extreme temperatures makes beryllium metal and aluminium beryllium alloys ideal for use in high performance aerospace applications such as components of rockets. Transparency to x-radiation makes pure beryllium metal essential in security equipment and high-resolution medical imaging technology, such as mammography to detect breast cancer. Copper beryllium is the hardest and strongest of any copper alloy (UTS up to 1,400 MPa), in the fully heat treated and cold worked condition. It combines high strength with non-magnetic and non-sparking qualities and it is similar in mechanical properties to many high strength alloy steels but, compared to steels, it has better corrosion resistance.
Chlorine
Chlorine is used in the manufacture of a wide range of consumer products, about two-thirds of them organic chemicals such as polyvinyl chloride (PVC), many intermediates for the production of plastics, and other end products which do not contain the element. As a common disinfectant, elemental chlorine and chlorine-generating compounds are used more directly in swimming pools to keep them sanitary. While perhaps best known for its role in providing clean drinking water, chlorine chemistry also helps provide energy-efficient building materials, electronics, fiber optics, solar energy cells, 93 percent of life-saving pharmaceuticals, 86 percent of crop protection compounds, medical plastics, and much more.
Beryllium and Chlorine – Comparison in Table
| Element | Beryllium | Chlorine |
| Density | 1.848 g/cm3 | 0.0032/cm3 |
| Ultimate Tensile Strength | 345 MPa | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | 287 GPa | N/A |
| Mohs Scale | 5.5 | N/A |
| Brinell Hardness | 600 MPa | N/A |
| Vickers Hardness | 1670 MPa | N/A |
| Melting Point | 1278 °C | -101 °C |
| Boiling Point | 2469 °C | -34.6 °C |
| Thermal Conductivity | 200 W/mK | 0.0089 W/mK |
| Thermal Expansion Coefficient | 11.3 µm/mK | — µm/mK |
| Specific Heat | 1.82 J/g K | 0.48 J/g K |
| Heat of Fusion | 12.2 kJ/mol | 3.23 kJ/mol |
| Heat of Vaporization | 292.4 kJ/mol | 10.2 kJ/mol |














