This article contains comparison of key thermal and atomic properties of chlorine and copper, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Chlorine vs Copper.
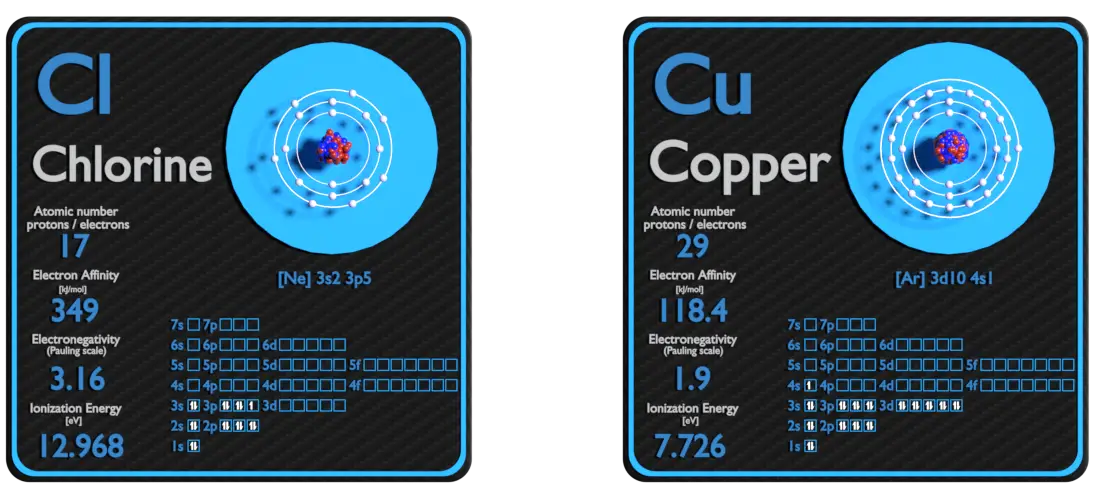
Chlorine and Copper – About Elements


Source: www.luciteria.com
Chlorine and Copper – Applications
Chlorine
Chlorine is used in the manufacture of a wide range of consumer products, about two-thirds of them organic chemicals such as polyvinyl chloride (PVC), many intermediates for the production of plastics, and other end products which do not contain the element. As a common disinfectant, elemental chlorine and chlorine-generating compounds are used more directly in swimming pools to keep them sanitary. While perhaps best known for its role in providing clean drinking water, chlorine chemistry also helps provide energy-efficient building materials, electronics, fiber optics, solar energy cells, 93 percent of life-saving pharmaceuticals, 86 percent of crop protection compounds, medical plastics, and much more.
Copper
Historically, alloying copper with another metal, for example tin to make bronze, was first practiced about 4000 years after the discovery of copper smelting, and about 2000 years after “natural bronze” had come into general use. An ancient civilization is defined to be in the Bronze Age either by producing bronze by smelting its own copper and alloying with tin, arsenic, or other metals. The major applications of copper are electrical wire (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is used mostly as a pure metal, but when greater hardness is required, it is put into such alloys as brass and bronze (5% of total use). Copper and copper-based alloys including brasses (Cu-Zn) and bronzes (Cu-Sn) are widely used in different industrial and societal applications. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Bronze, or bronze-like alloys and mixtures, were used for coins over a longer period. is still widely used today for springs, bearings, bushings, automobile transmission pilot bearings, and similar fittings, and is particularly common in the bearings of small electric motors. Brass and bronze are common engineering materials in modern architecture and primarily used for roofing and facade cladding due to their visual appearance.
Chlorine and Copper – Comparison in Table
| Element | Chlorine | Copper |
| Density | 0.0032 g/cm3 | 8.92 g/cm3 |
| Ultimate Tensile Strength | N/A | 210 MPa |
| Yield Strength | N/A | 33 MPa |
| Young’s Modulus of Elasticity | N/A | 120 GPa |
| Mohs Scale | N/A | 3 |
| Brinell Hardness | N/A | 250 MPa |
| Vickers Hardness | N/A | 350 MPa |
| Melting Point | -101 °C | 1084.62 °C |
| Boiling Point | -34.6 °C | 2562 °C |
| Thermal Conductivity | 0.0089 W/mK | 401 W/mK |
| Thermal Expansion Coefficient | N/A | 16.5 µm/mK |
| Specific Heat | 0.48 J/g K | 0.38 J/g K |
| Heat of Fusion | 3.23 kJ/mol | 13.05 kJ/mol |
| Heat of Vaporization | 10.2 kJ/mol | 300.3 kJ/mol |






















