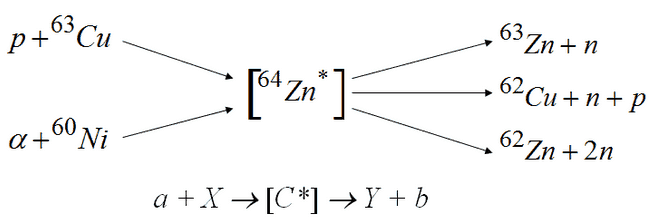Key Characteristics of Inelastic Scattering
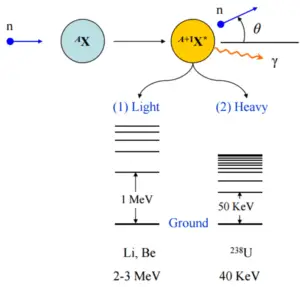
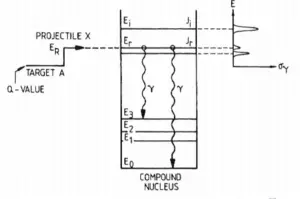
- During an inelastic scattering the neutron is absorbed and then re-emitted.
- The reaction occurs via compound nucleus.
- While momentum is conserved in an inelastic collision, kinetic energy of the “system” is not conserved.
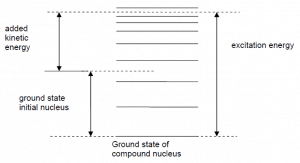
- Some energy of the incident neutron is absorbed to the recoiling nucleus and the nucleus remains in the excited state.
- The nucleus gives up excitation energy by emitting one or more gamma rays.
- General notation: A(n, n’)A* or A(n, 2n’)B; Example: 14O(n, n’)14O*.
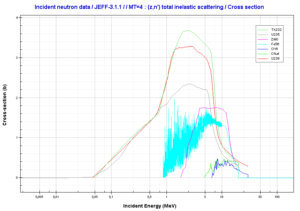
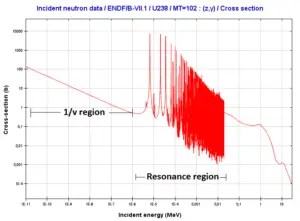
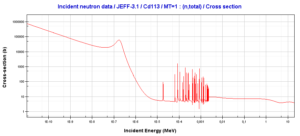
- Inelastic scattering is a threshold reaction and occurs above a threshold energy.
- Inelastic scattering cross section is relatively small for light nuclei.
- For hydrogen nucleus, inelastic scattering does not occur, because it does not have excited states.
- Inelastic scattering plays an important role in slowing down neutrons especially at high energies and by heavy nuclei (e.g. 238U).
- Inelastic scattering may be significant for heterogeneous reactors with highly enriched fuel (e.g. in fast neutron reactors).
We hope, this article, Key Characteristics of Inelastic Scattering, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.