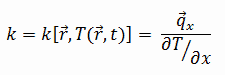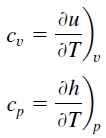About Graphene
Graphene is an allotrope of carbon consisting of a single layer of atoms hexagonally arranged in a two-dimensional lattice. Graphene is a substance with very interesting properties. Graphene has high thermal conductivity, high electrical conductivity, high elasticity and flexibility, high hardness and strength. These properties, together with the abundance of carbon in nature, have made graphene a very studied material with great possibilities.
Summary
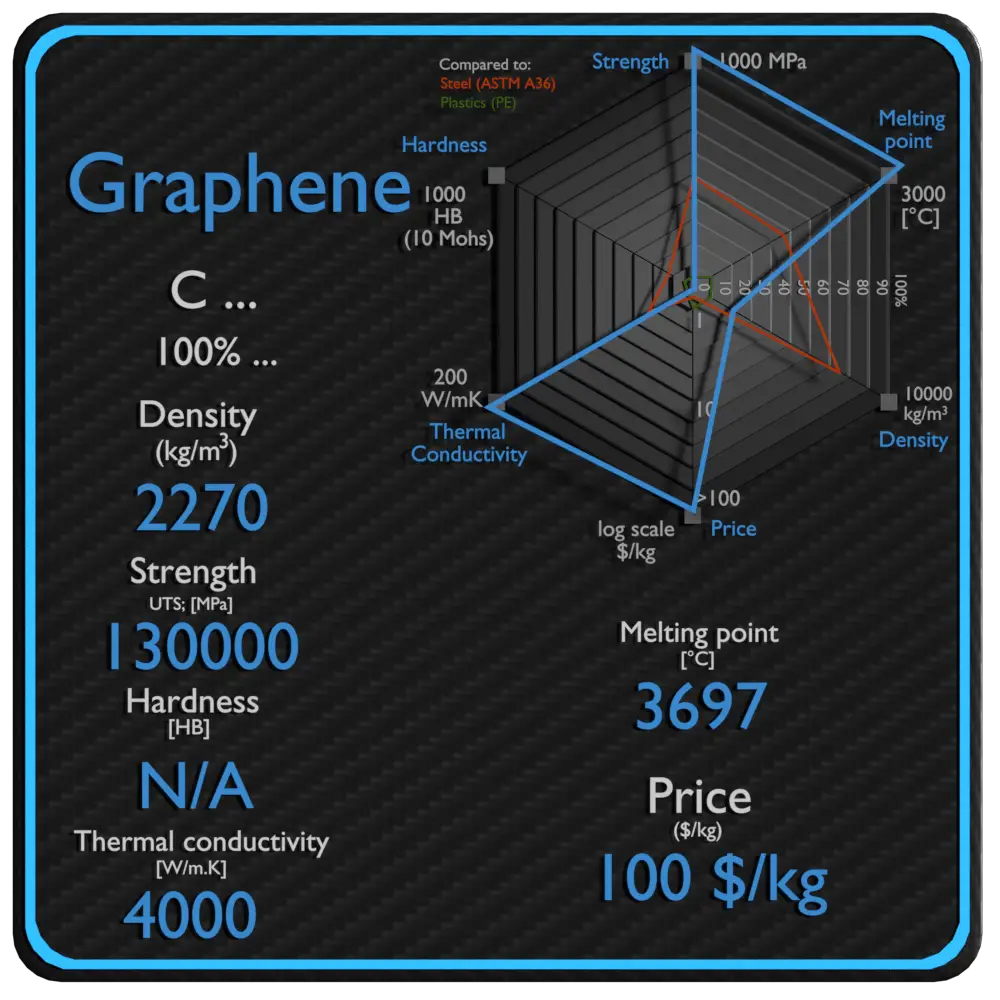
| Name | Graphene |
| Phase at STP | solid |
| Density | 2270 kg/m3 |
| Ultimate Tensile Strength | 130000 MPa |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | 1000 GPa |
| Brinell Hardness | N/A |
| Melting Point | 3697 °C |
| Thermal Conductivity | 4000 W/mK |
| Heat Capacity | N/A |
| Price | 100 $/kg |
Composition of Graphene
Graphene is a material composed of carbon atoms grouping that are hexagonally positioned. This arrangement results in monolayers of an atom thick. Each atom in a graphene sheet is connected to its three nearest neighbors by a σ-bond, and contributes one electron to a conduction band that extends over the whole sheet.
Applications of Graphene
Graphene is a transparent and flexible conductor that holds great promise for various material/device applications, including solar cells, light-emitting diodes (LED), touch panels, and smart windows or phones. Smartphone products with graphene touch screens are already on the market. However, with existing manufacturing processes Graphene is extremely challenging to mass produce which could prove to be a limiting factor in its commercial use.
Mechanical Properties of Graphene
Strength of Graphene
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. In designing structures and machines, it is important to consider these factors, in order that the material selected will have adequate strength to resist applied loads or forces and retain its original shape.
Strength of a material is its ability to withstand this applied load without failure or plastic deformation. For tensile stress, the capacity of a material or structure to withstand loads tending to elongate is known as ultimate tensile strength (UTS). Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins. In case of tensional stress of a uniform bar (stress-strain curve), the Hooke’s law describes behaviour of a bar in the elastic region. The Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests.
See also: Strength of Materials
Ultimate Tensile Strength of Graphene
Ultimate tensile strength of Graphene is 130000 MPa.
Yield Strength of Graphene
Yield strength of Graphene is N/A.
Modulus of Elasticity of Graphene
The Young’s modulus of elasticity of Graphene is 1000 GPa.
Hardness of Graphene
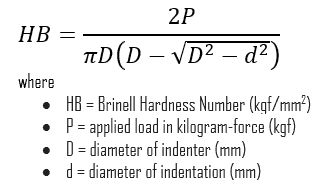
In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Brinell hardness test is one of indentation hardness tests, that has been developed for hardness testing. In Brinell tests, a hard, spherical indenter is forced under a specific load into the surface of the metal to be tested.
The Brinell hardness number (HB) is the load divided by the surface area of the indentation. The diameter of the impression is measured with a microscope with a superimposed scale. The Brinell hardness number is computed from the equation:
Brinell hardness of Graphene is approximately N/A.
See also: Hardness of Materials
Thermal Properties of Graphene
Graphene – Melting Point
Melting point of Graphene is 3697 °C.
Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. For various chemical compounds and alloys, it is difficult to define the melting point, since they are usually a mixture of various chemical elements.
Graphene – Thermal Conductivity
Thermal conductivity of Graphene is 4000 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Graphene – Specific Heat
Specific heat of Graphene is 509 J/g K.
Specific heat, or specific heat capacity, is a property related to internal energy that is very important in thermodynamics. The intensive properties cv and cp are defined for pure, simple compressible substances as partial derivatives of the internal energy u(T, v) and enthalpy h(T, p), respectively:
where the subscripts v and p denote the variables held fixed during differentiation. The properties cv and cp are referred to as specific heats (or heat capacities) because under certain special conditions they relate the temperature change of a system to the amount of energy added by heat transfer. Their SI units are J/kg K or J/mol K.
Properties and prices of other materials
material-table-in-8k-resolution