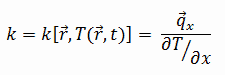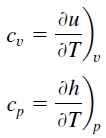About Limestone
Limestone is very common in architecture, especially in Europe and North America. Limestone is extracted from quarries or mines. Part of the extracted stone, selected according to its chemical composition and optical granulometry, is calcinated at about 1,000 °C (1,830 °F) in different types of lime kilns to produce quicklime. The principal users of lime are steelmaking industry (lowering slag melting temperature) ~ 35 %, environmental uses (desulfurization, water cleaning) ~ 20 %, civil engineering ~ 20 % and chemistry ~ 8 %.
Summary
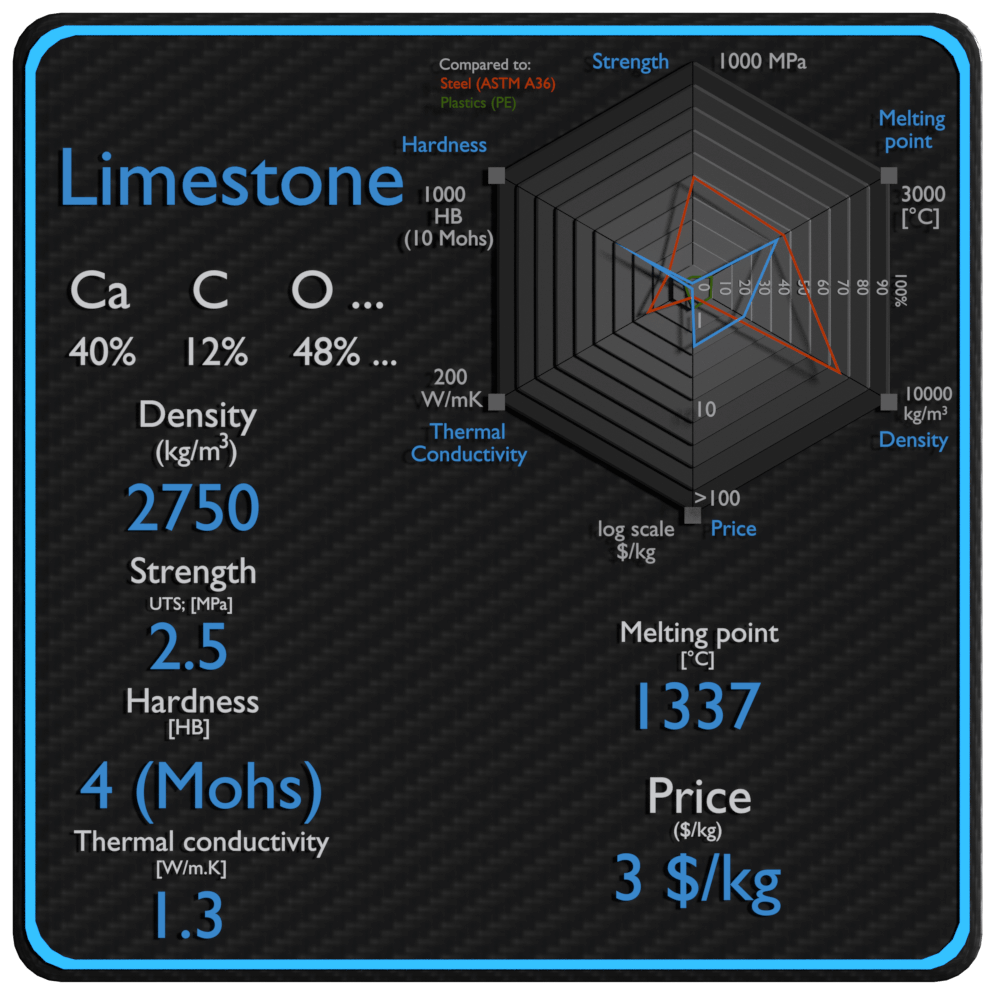
| Name | Limestone |
| Phase at STP | solid |
| Density | 2750 kg/m3 |
| Ultimate Tensile Strength | 2.5 MPa |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | 34 GPa |
| Brinell Hardness | 4 Mohs |
| Melting Point | 1337 °C |
| Thermal Conductivity | 1.3 W/mK |
| Heat Capacity | 840 J/g K |
| Price | 3 $/kg |
Composition of Limestone
The main component of limestone is calcium carbonate (CaCO3), also known as calcite, which is formed by the compaction of the remains of coral animals and plants on the bottoms of oceans. It varies from a soft white substance (chalk) to a very hard substance (marble).
Applications of Limestone

Limestone is very common in architecture, especially in Europe and North America. Limestone is extracted from quarries or mines. Part of the extracted stone, selected according to its chemical composition and optical granulometry, is calcinated at about 1,000 °C (1,830 °F) in different types of lime kilns to produce quicklime. The principal users of lime are steelmaking industry (lowering slag melting temperature) ~ 35 %, environmental uses (desulfurization, water cleaning) ~ 20 %, civil engineering ~ 20 % and chemistry ~ 8 %.
Mechanical Properties of Limestone
Strength of Limestone
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. In designing structures and machines, it is important to consider these factors, in order that the material selected will have adequate strength to resist applied loads or forces and retain its original shape.
Strength of a material is its ability to withstand this applied load without failure or plastic deformation. For tensile stress, the capacity of a material or structure to withstand loads tending to elongate is known as ultimate tensile strength (UTS). Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins. In case of tensional stress of a uniform bar (stress-strain curve), the Hooke’s law describes behaviour of a bar in the elastic region. The Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests.
See also: Strength of Materials
Ultimate Tensile Strength of Limestone
Ultimate tensile strength of Limestone is 2.5 MPa.
Yield Strength of Limestone
Yield strength of Limestone is N/A.
Modulus of Elasticity of Limestone
The Young’s modulus of elasticity of Limestone is 34 GPa.
Hardness of Limestone
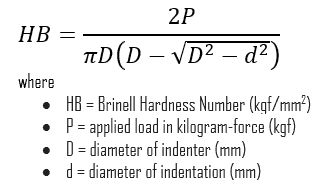
In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Brinell hardness test is one of indentation hardness tests, that has been developed for hardness testing. In Brinell tests, a hard, spherical indenter is forced under a specific load into the surface of the metal to be tested.
The Brinell hardness number (HB) is the load divided by the surface area of the indentation. The diameter of the impression is measured with a microscope with a superimposed scale. The Brinell hardness number is computed from the equation:
Hardness of Limestone is approximately 4 Mohs.
See also: Hardness of Materials
Thermal Properties of Limestone
Limestone – Melting Point
Melting point of Limestone is 1337 °C.
Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. For various chemical compounds and alloys, it is difficult to define the melting point, since they are usually a mixture of various chemical elements.
Limestone – Thermal Conductivity
Thermal conductivity of Limestone is 1.3 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Limestone – Specific Heat
Specific heat of Limestone is 840 J/g K.
Specific heat, or specific heat capacity, is a property related to internal energy that is very important in thermodynamics. The intensive properties cv and cp are defined for pure, simple compressible substances as partial derivatives of the internal energy u(T, v) and enthalpy h(T, p), respectively:
where the subscripts v and p denote the variables held fixed during differentiation. The properties cv and cp are referred to as specific heats (or heat capacities) because under certain special conditions they relate the temperature change of a system to the amount of energy added by heat transfer. Their SI units are J/kg K or J/mol K.
Properties and prices of other materials
material-table-in-8k-resolution