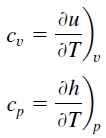About Osmium
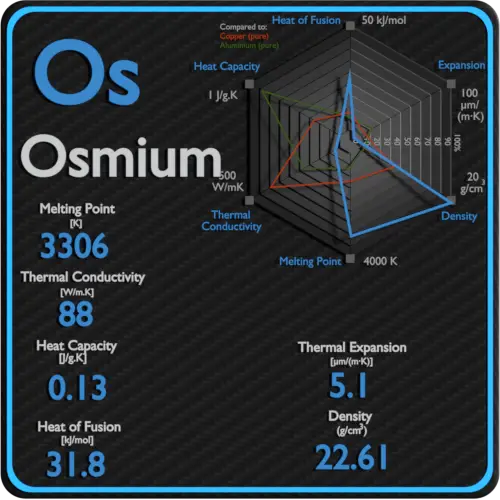
Osmium is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element, with a density of 22.59 g/cm3. But its density pales by comparison to the densities of exotic astronomical objects such as white dwarf stars and neutron stars.
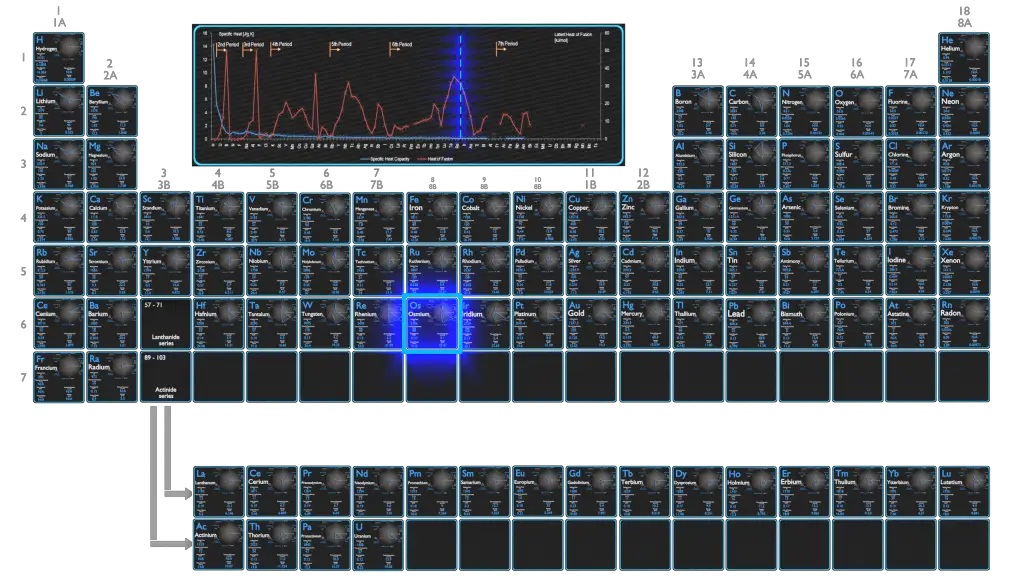
Osmium – Specific Heat, Latent Heat of Fusion, Latent Heat of Vaporization
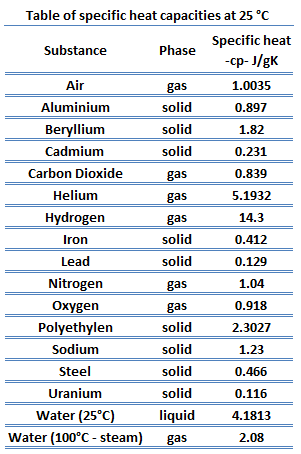
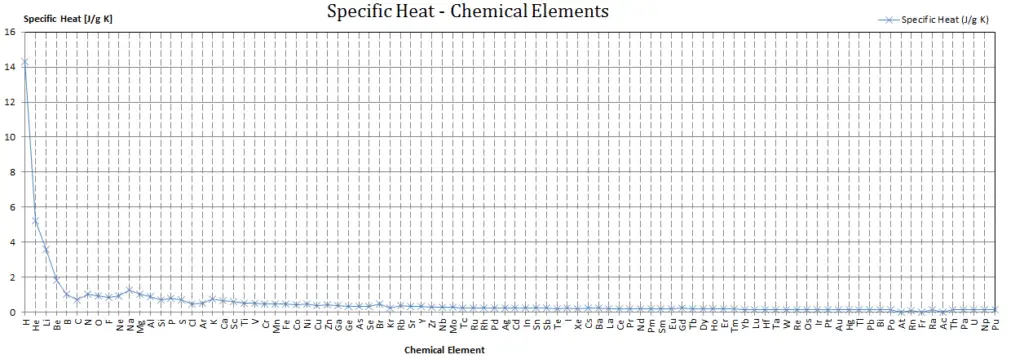
Specific heat of Osmium is 0.13 J/g K.
Heat capacity is an extensive property of matter, meaning it is proportional to the size of the system. Heat capacity C has the unit of energy per degree or energy per kelvin. When expressing the same phenomenon as an intensive property, the heat capacity is divided by the amount of substance, mass, or volume, thus the quantity is independent of the size or extent of the sample.
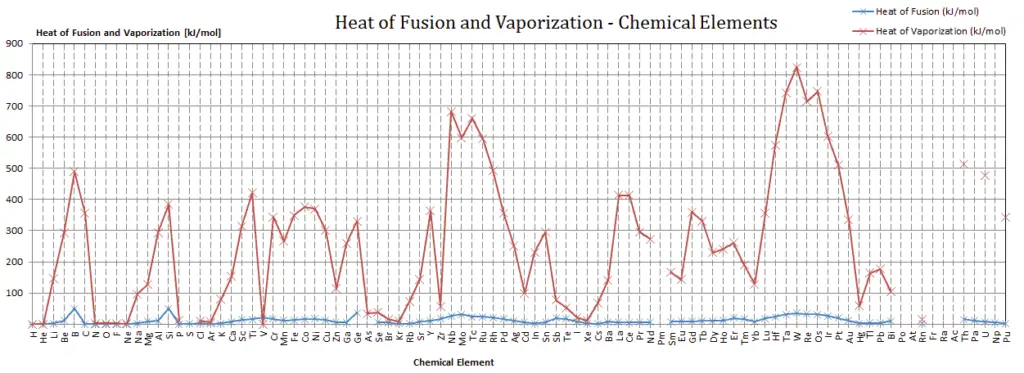
Latent Heat of Fusion of Osmium is 31.8 kJ/mol.
Latent Heat of Vaporization of Osmium is 746 kJ/mol.
Latent heat is the amount of heat added to or removed from a substance to produce a change in phase. This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the gas (the pΔV work). When latent heat is added, no temperature change occurs. The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
See also: Mechanical Properties of Osmium
Summary
| Element | Osmium |
| Specific Heat | 0.13 J/g K |
| Heat of Fusion | 31.8 kJ/mol |
| Heat of Vaporization | 746 kJ/mol |
| Density | 22.61 g/cm3 |
Source: www.luciteria.com