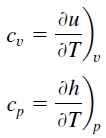About Beryllium
Beryllium is a hard, grayish metal naturally found in mineral rocks, coal, soil, and volcanic dust. The commercial use of beryllium requires the use of appropriate dust control equipment and industrial controls at all times because of the toxicity of inhaled beryllium-containing dusts that can cause a chronic life-threatening allergic disease in some people called berylliosis. Beryllium has a large scattering cross section for high-energy neutrons, about 6 barns for energies above approximately 10 keV. Therefore, it works as a neutron reflector and neutron moderator, effectively slowing the neutrons to the thermal energy. Since berylium has very low threshold energy for neutron emission, it can be used as a neutron source in nuclear reactors. The Sb-Be source is based on (γ,n) reaction (i.e. it emits photoneutrons).
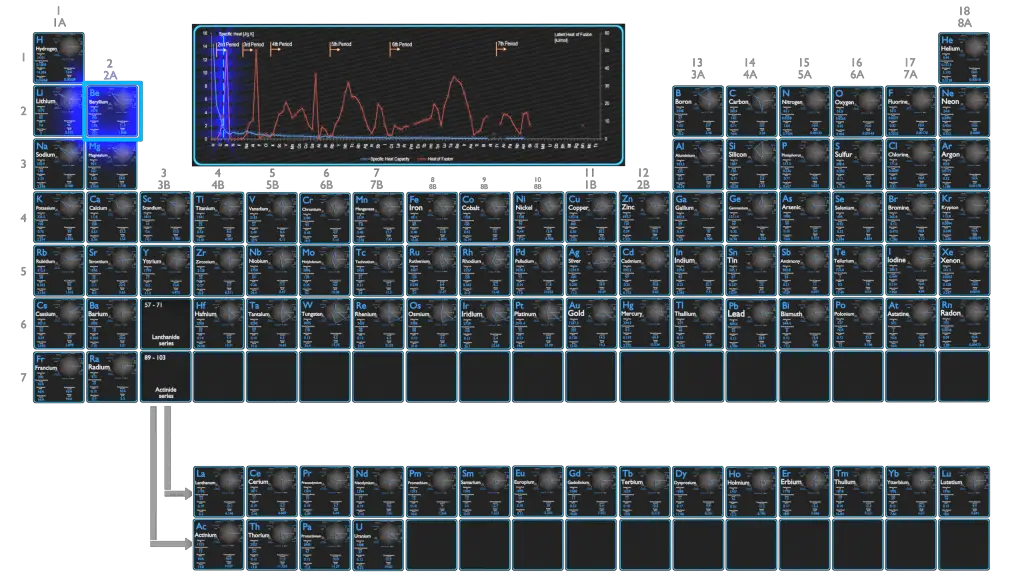
Beryllium – Specific Heat, Latent Heat of Fusion, Latent Heat of Vaporization
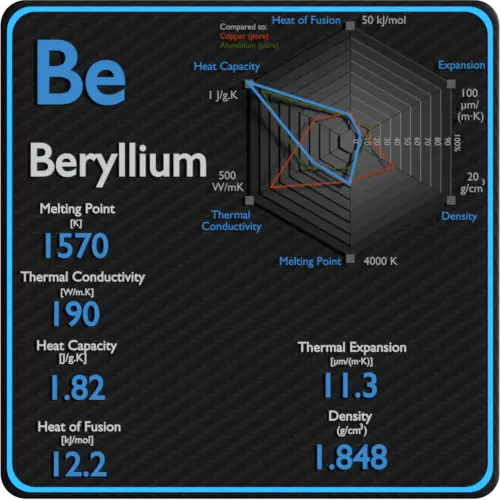
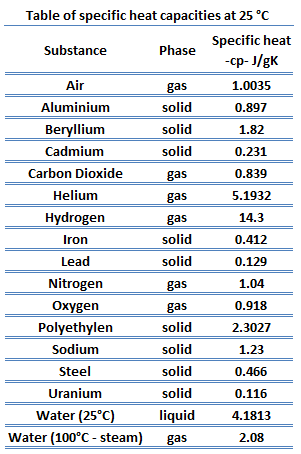
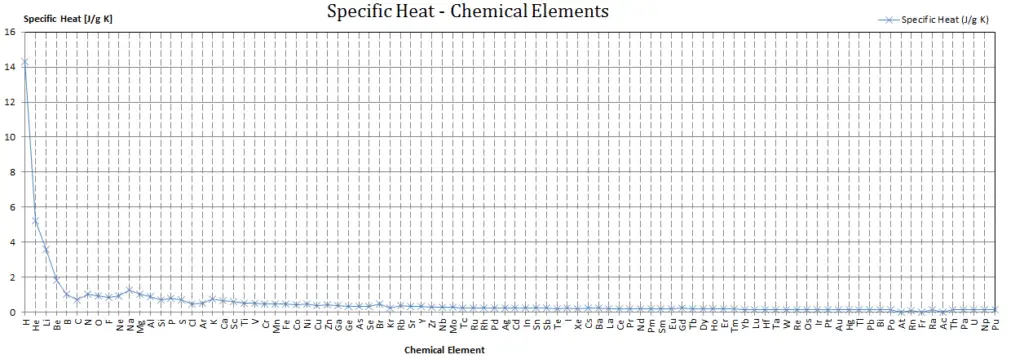
Specific heat of Beryllium is 1.82 J/g K.
Heat capacity is an extensive property of matter, meaning it is proportional to the size of the system. Heat capacity C has the unit of energy per degree or energy per kelvin. When expressing the same phenomenon as an intensive property, the heat capacity is divided by the amount of substance, mass, or volume, thus the quantity is independent of the size or extent of the sample.
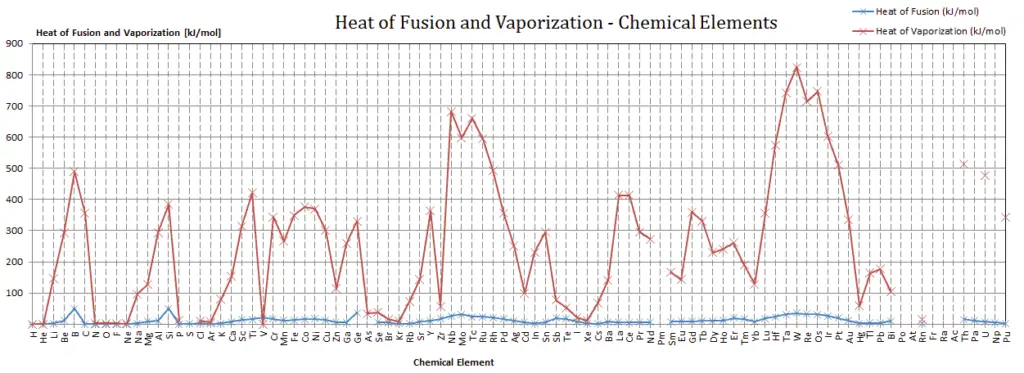
Latent Heat of Fusion of Beryllium is 12.2 kJ/mol.
Latent Heat of Vaporization of Beryllium is 292.4 kJ/mol.
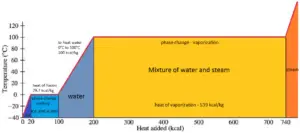
Latent heat is the amount of heat added to or removed from a substance to produce a change in phase. This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the gas (the pΔV work). When latent heat is added, no temperature change occurs. The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
See also: Mechanical Properties of Beryllium
Summary
| Element | Beryllium |
| Specific Heat | 1.82 J/g K |
| Heat of Fusion | 12.2 kJ/mol |
| Heat of Vaporization | 292.4 kJ/mol |
| Density | 1.848 g/cm3 |
Source: www.luciteria.com