This article contains comparison of key thermal and atomic properties of selenium and iodine, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Selenium vs Iodine.
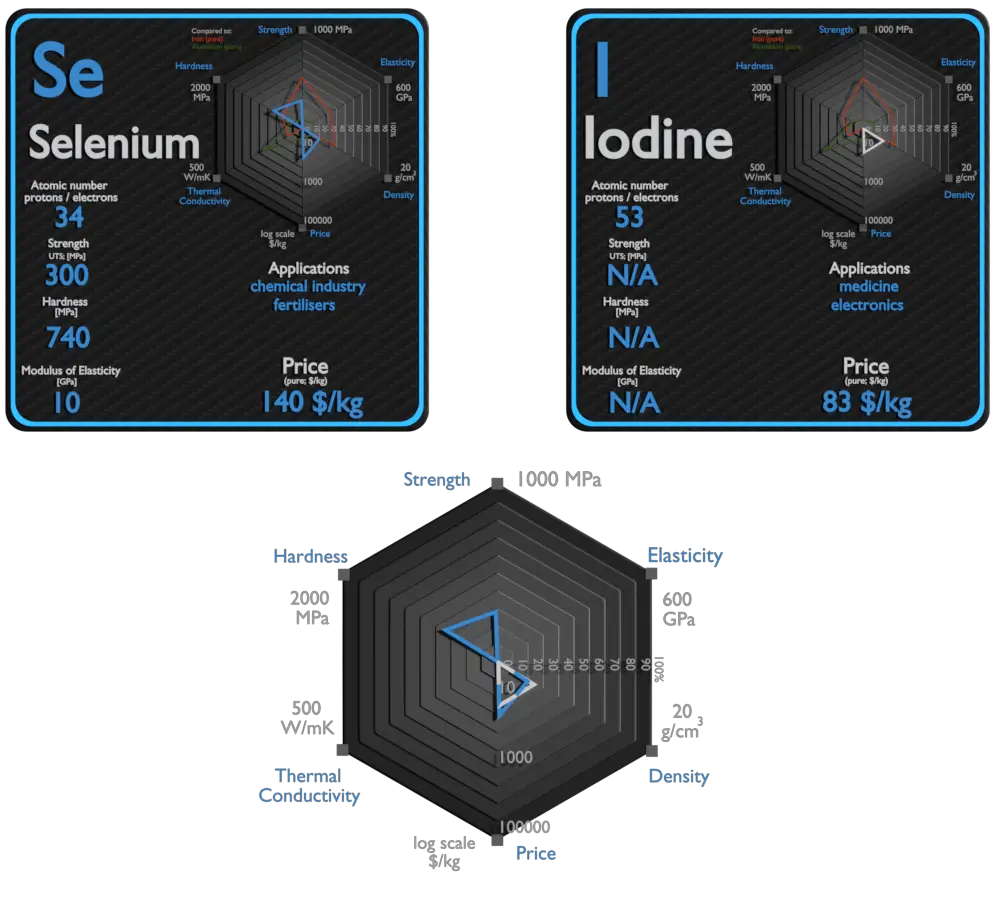
Selenium and Iodine – About Elements


Source: www.luciteria.com
Selenium and Iodine – Applications
Selenium
The chief commercial uses for selenium today are glassmaking and pigments. Selenium finds applications in the various industries, for example, solar cells and photoconductor applications, manganese electrolysis, DC power surge protection or X-ray crystallography.
Iodine
In addition to nutrition products, iodine and iodine derivatives are used in a wide range of medical, agricultural, and industrial applications. About half of all produced iodine goes into various organoiodine compounds, another 15% remains as the pure element, another 15% is used to form potassium iodide, and another 15% for other inorganic iodine compounds. The leading application is in the production of X-ray contrast media (22%). Iodine’s high atomic number and density make it ideally suited for this application, as its presence in the body can help to increase contrast between tissues, organs, and blood vessels with similar X-ray densities. It is used as an antiseptic for external wounds. Another application driving the demand for iodine is in polarizing film in liquidcrystal display (LCD) screens.
Selenium and Iodine – Comparison in Table
| Element | Selenium | Iodine |
| Density | 4.819 g/cm3 | 4.94 g/cm3 |
| Ultimate Tensile Strength | 300 MPa | N/A |
| Yield Strength | 150 MPa | N/A |
| Young’s Modulus of Elasticity | 10 GPa | N/A |
| Mohs Scale | 2 | N/A |
| Brinell Hardness | 740 MPa | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | 221 °C | 113.5 °C |
| Boiling Point | 685 °C | 184 °C |
| Thermal Conductivity | 2.04 W/mK | 0.449 W/mK |
| Thermal Expansion Coefficient | 37 µm/mK | N/A |
| Specific Heat | 0.32 J/g K | 0.214 J/g K |
| Heat of Fusion | 6.694 kJ/mol | 7.824 kJ/mol |
| Heat of Vaporization | 37.7 kJ/mol | 20.752 kJ/mol |








