This article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Chlorine vs Iodine.
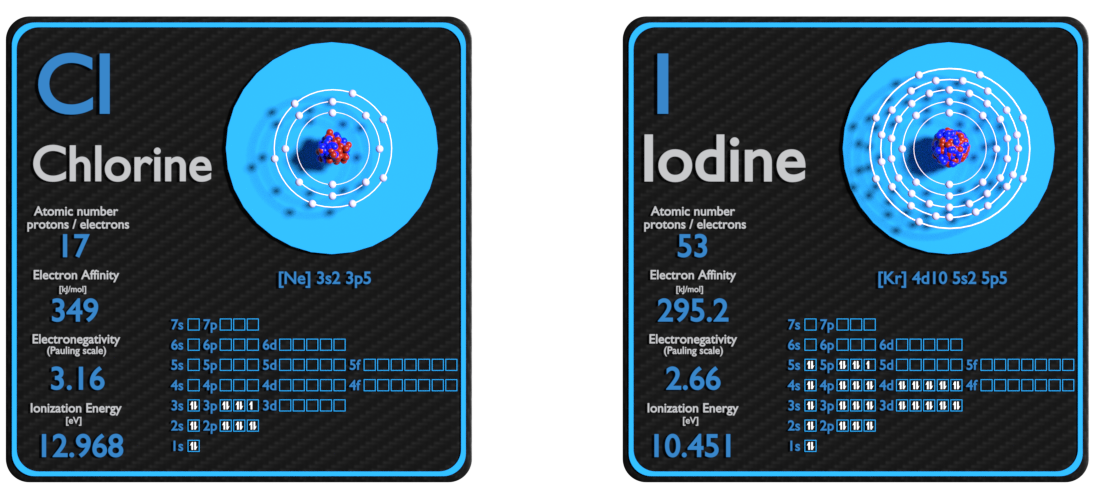
Chlorine and Iodine – About Elements


Source: www.luciteria.com
Chlorine and Iodine – Applications
Chlorine
Chlorine is used in the manufacture of a wide range of consumer products, about two-thirds of them organic chemicals such as polyvinyl chloride (PVC), many intermediates for the production of plastics, and other end products which do not contain the element. As a common disinfectant, elemental chlorine and chlorine-generating compounds are used more directly in swimming pools to keep them sanitary. While perhaps best known for its role in providing clean drinking water, chlorine chemistry also helps provide energy-efficient building materials, electronics, fiber optics, solar energy cells, 93 percent of life-saving pharmaceuticals, 86 percent of crop protection compounds, medical plastics, and much more.
Iodine
In addition to nutrition products, iodine and iodine derivatives are used in a wide range of medical, agricultural, and industrial applications. About half of all produced iodine goes into various organoiodine compounds, another 15% remains as the pure element, another 15% is used to form potassium iodide, and another 15% for other inorganic iodine compounds. The leading application is in the production of X-ray contrast media (22%). Iodine’s high atomic number and density make it ideally suited for this application, as its presence in the body can help to increase contrast between tissues, organs, and blood vessels with similar X-ray densities. It is used as an antiseptic for external wounds. Another application driving the demand for iodine is in polarizing film in liquidcrystal display (LCD) screens.
Chlorine and Iodine – Comparison in Table
| Element | Chlorine | Iodine |
| Density | 0.0032 g/cm3 | 4.94 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -101 °C | 113.5 °C |
| Boiling Point | -34.6 °C | 184 °C |
| Thermal Conductivity | 0.0089 W/mK | 0.449 W/mK |
| Thermal Expansion Coefficient | N/A | N/A |
| Specific Heat | 0.48 J/g K | 0.214 J/g K |
| Heat of Fusion | 3.23 kJ/mol | 7.824 kJ/mol |
| Heat of Vaporization | 10.2 kJ/mol | 20.752 kJ/mol |




















