This article contains comparison of key thermal and atomic properties of zinc and selenium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Zinc vs Selenium.
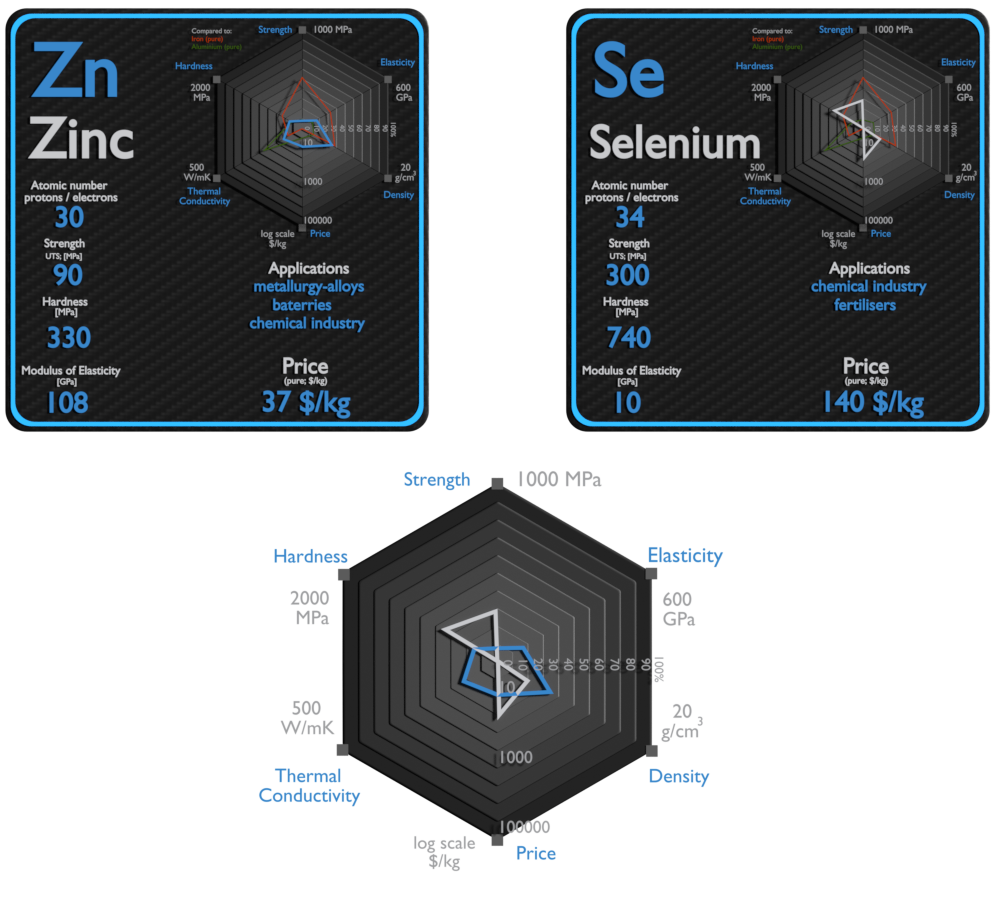
Zinc and Selenium – About Elements


Source: www.luciteria.com
Zinc and Selenium – Applications
Zinc
Corrosion-resistant zinc plating of iron (hot-dip galvanizing) is the major application for zinc. Coating of steel constitutes the largest single use of zinc, but it is used in large tonnages in zinc alloy castings, as zinc dust and oxide, and in wrought zinc products. Galvanized steel is just plain carbon steel that has been coated with a thin zinc layer. The zinc protects iron by corroding first, but zinc corrodes at much lower rates than do steel. Other applications are in electrical batteries, small non-structural castings, and alloys such as brass. A variety of zinc compounds are commonly used, such as zinc carbonate and zinc gluconate (as dietary supplements), zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and dimethylzinc or diethylzinc in the organic laboratory. A key part of the modern materials world in which zinc finds itself is recycling. Zinc, in common with all metals (and unlike synthetic materials) can be recycled indefinitely without degradation.
Selenium
The chief commercial uses for selenium today are glassmaking and pigments. Selenium finds applications in the various industries, for example, solar cells and photoconductor applications, manganese electrolysis, DC power surge protection or X-ray crystallography.
Zinc and Selenium – Comparison in Table
| Element | Zinc | Selenium |
| Density | 7.14 g/cm3 | 4.819 g/cm3 |
| Ultimate Tensile Strength | 90 MPa | 300 MPa |
| Yield Strength | 75 MPa | 150 MPa |
| Young’s Modulus of Elasticity | 108 GPa | 10 GPa |
| Mohs Scale | 2.5 | 2 |
| Brinell Hardness | 330 MPa | 740 MPa |
| Vickers Hardness | N/A | N/A |
| Melting Point | 419.53 °C | 221 °C |
| Boiling Point | 907 °C | 685 °C |
| Thermal Conductivity | 116 W/mK | 2.04 W/mK |
| Thermal Expansion Coefficient | 30.2 µm/mK | 37 µm/mK |
| Specific Heat | 0.39 J/g K | 0.23 J/g K |
| Heat of Fusion | 7.322 kJ/mol | 6.694 kJ/mol |
| Heat of Vaporization | 115.3 kJ/mol | 37.7 kJ/mol |











