This article contains comparison of key thermal and atomic properties of iron and zinc, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Iron vs Zinc.
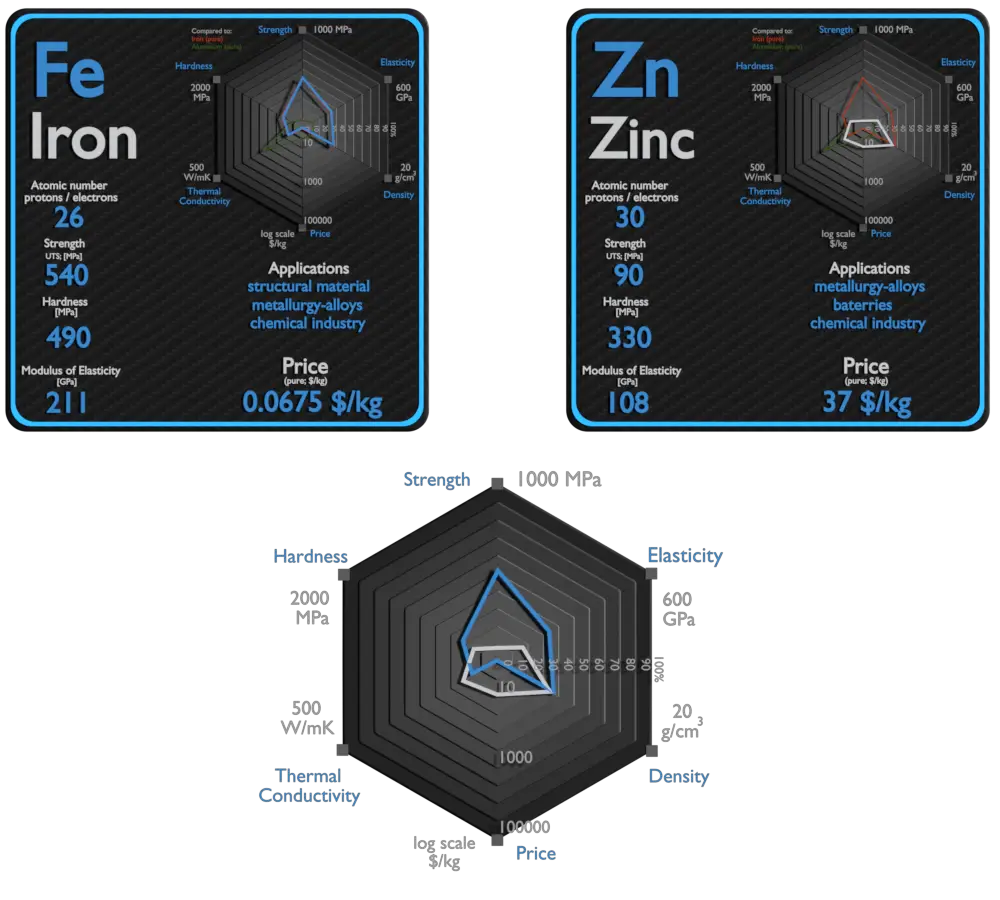
Iron and Zinc – About Elements


Source: www.luciteria.com
Iron and Zinc – Applications
Iron
Iron is used in numerous sectors such as electronics, manufacturing, automotive, and construction and building. Iron is the most widely used of all the metals, accounting for over 90% of worldwide metal produc0tion. Its low cost and high strength often make it the material of choice material to withstand stress or transmit forces, such as the construction of machinery and machine tools, rails, automobiles, ship hulls, concrete reinforcing bars, and the load-carrying framework of buildings. Since pure iron is quite soft, it is most commonly combined with alloying elements to make steel. Steels are iron–carbon alloys that may contain appreciable concentrations of other alloying elements. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Due to its very-high strength, but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloy in modern use. There are thousands of alloys that have different compositions and/or heat treatments. The mechanical properties are sensitive to the content of carbon, which is normally less than 1.0 wt%.
Zinc
Corrosion-resistant zinc plating of iron (hot-dip galvanizing) is the major application for zinc. Coating of steel constitutes the largest single use of zinc, but it is used in large tonnages in zinc alloy castings, as zinc dust and oxide, and in wrought zinc products. Galvanized steel is just plain carbon steel that has been coated with a thin zinc layer. The zinc protects iron by corroding first, but zinc corrodes at much lower rates than do steel. Other applications are in electrical batteries, small non-structural castings, and alloys such as brass. A variety of zinc compounds are commonly used, such as zinc carbonate and zinc gluconate (as dietary supplements), zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and dimethylzinc or diethylzinc in the organic laboratory. A key part of the modern materials world in which zinc finds itself is recycling. Zinc, in common with all metals (and unlike synthetic materials) can be recycled indefinitely without degradation.
Iron and Zinc – Comparison in Table
| Element | Iron | Zinc |
| Density | 7.874 g/cm3 | 7.14 g/cm3 |
| Ultimate Tensile Strength | 540 MPa | 90 MPa |
| Yield Strength | 50 MPa | 75 MPa |
| Young’s Modulus of Elasticity | 211 GPa | 108 GPa |
| Mohs Scale | 4.5 | 2.5 |
| Brinell Hardness | 490 MPa | 330 MPa |
| Vickers Hardness | 608 MPa | N/A |
| Melting Point | 1538 °C | 419.53 °C |
| Boiling Point | 2861 °C | 907 °C |
| Thermal Conductivity | 80.2 W/mK | 116 W/mK |
| Thermal Expansion Coefficient | 11.8 µm/mK | 30.2 µm/mK |
| Specific Heat | 0.44 J/g K | 0.39 J/g K |
| Heat of Fusion | 13.8 kJ/mol | 7.322 kJ/mol |
| Heat of Vaporization | 349.6 kJ/mol | 115.3 kJ/mol |



















