This article contains comparison of key thermal and atomic properties of helium and neon, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Helium vs Neon.
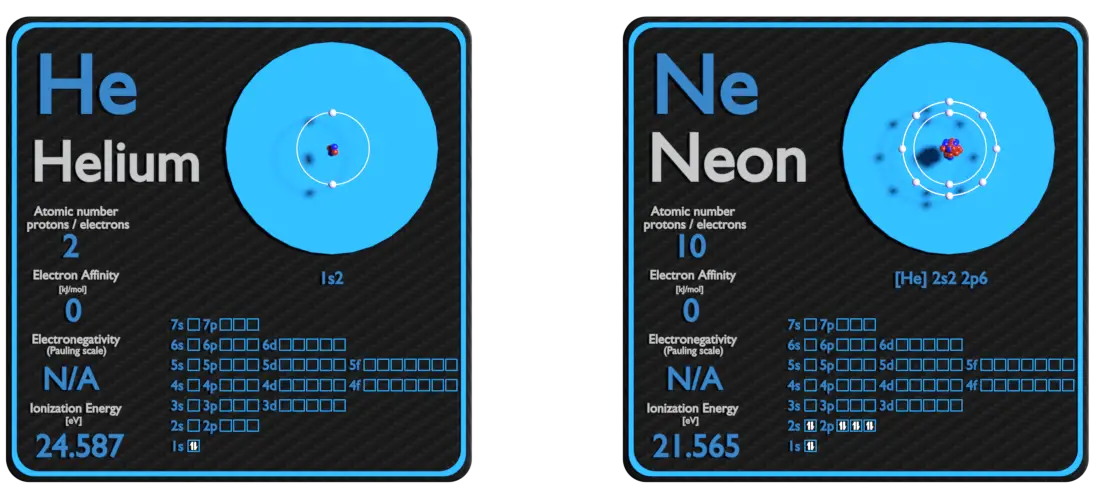
Helium and Neon – About Elements


Source: www.luciteria.com
Helium and Neon – Applications
Helium
Helium is used for many purposes that require some of its unique properties, such as its low boiling point, low density, low solubility, high thermal conductivity, or inertness. Of the 2014 world helium total production of about 32 million kg (180 million standard cubic meters) helium per year, the largest use (about 32% of the total in 2014) is in cryogenic applications, most of which involves cooling the superconducting magnets in medical MRI scanners and NMR spectrometers. Most clinical magnets are superconducting magnets, which require liquid helium to keep them very cold.
Neon
Neon is often used in signs and produces an unmistakable bright reddish-orange light. Although tube lights with other colors are often called “neon”, they use different noble gases or varied colors of fluorescent lighting. Neon is also used to make high-voltage indicators and switching gear, lightning arresters, diving equipment and lasers. Liquid neon is an important cryogenic refrigerant. It has over 40 times more refrigerating capacity per unit volume than liquid helium, and more than 3 times that of liquid hydrogen
Helium and Neon – Comparison in Table
| Element | Helium | Neon |
| Density | 0.00018 g/cm3 | 0.0009 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -272.2 °C | -248 °C |
| Boiling Point | -268.9 °C | -248.7 °C |
| Thermal Conductivity | 0.1513 W/mK | 0.0493 W/mK |
| Thermal Expansion Coefficient | — µm/mK | — µm/mK |
| Specific Heat | 5.193 J/g K | 0.904 J/g K |
| Heat of Fusion | 0.0138 kJ/mol | 0.3317 kJ/mol |
| Heat of Vaporization | 0.0845 kJ/mol | 1.7326 kJ/mol |







