This article contains comparison of key thermal and atomic properties of helium and xenon, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Helium vs Xenon.
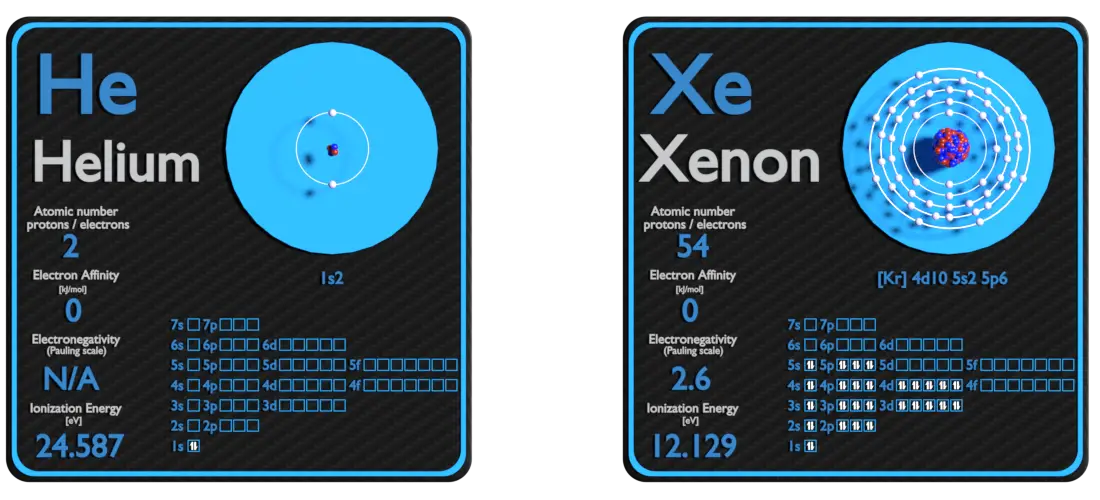
Helium and Xenon – About Elements


Source: www.luciteria.com
Helium and Xenon – Applications
Helium
Helium is used for many purposes that require some of its unique properties, such as its low boiling point, low density, low solubility, high thermal conductivity, or inertness. Of the 2014 world helium total production of about 32 million kg (180 million standard cubic meters) helium per year, the largest use (about 32% of the total in 2014) is in cryogenic applications, most of which involves cooling the superconducting magnets in medical MRI scanners and NMR spectrometers. Most clinical magnets are superconducting magnets, which require liquid helium to keep them very cold.
Xenon
Xenon is useful in the following applications. The white flash of light produced by xenon makes it suitable for usage in strobe lights and to power ruby lasers. Xenon is used in light-emitting devices called xenon flash lamps, used in photographic flashes and stroboscopic lamps.
Helium and Xenon – Comparison in Table
| Element | Helium | Xenon |
| Density | 0.00018 g/cm3 | 0.0059 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | N/A |
| Mohs Scale | N/A | N/A |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -272.2 °C | -111.8 °C |
| Boiling Point | -268.9 °C | -107.1 °C |
| Thermal Conductivity | 0.1513 W/mK | 0.00565 W/mK |
| Thermal Expansion Coefficient | — µm/mK | — µm/mK |
| Specific Heat | 5.193 J/g K | 0.158 J/g K |
| Heat of Fusion | 0.0138 kJ/mol | 2.297 kJ/mol |
| Heat of Vaporization | 0.0845 kJ/mol | 12.636 kJ/mol |







