This article contains comparison of key thermal and atomic properties of iridium and platinum, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Iridium vs Platinum.
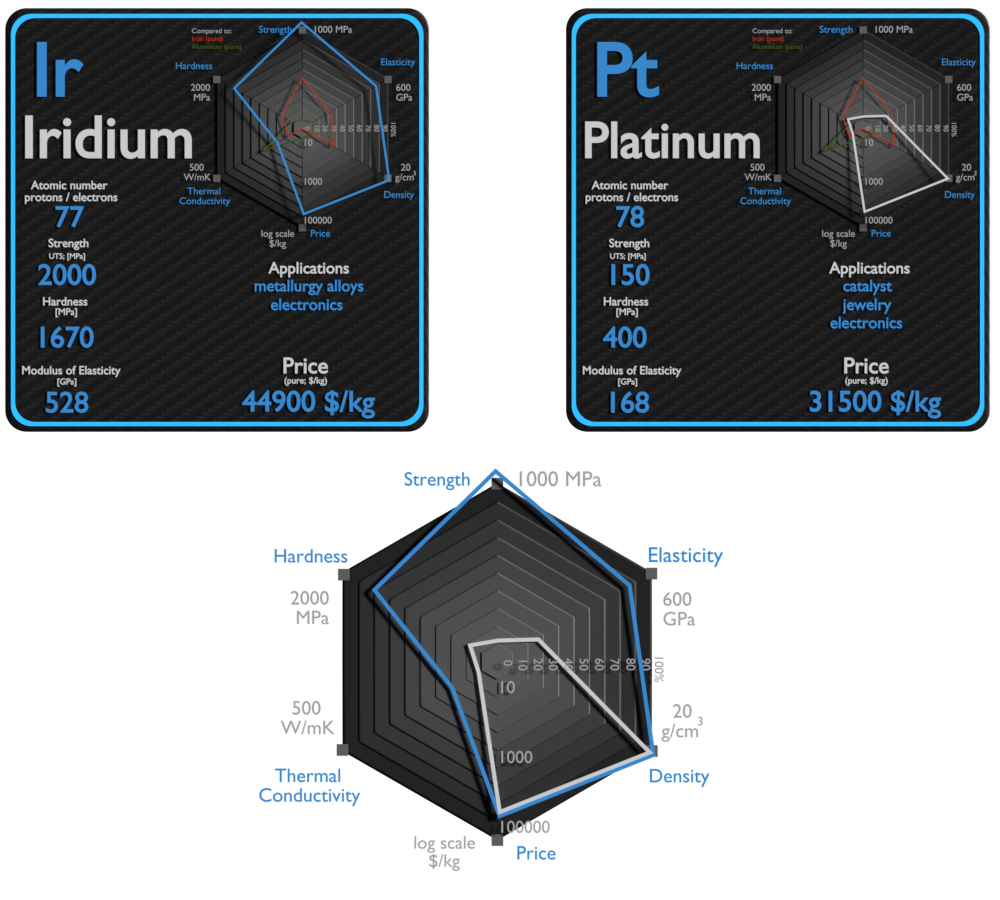
Iridium and Platinum – About Elements


Source: www.luciteria.com
Iridium and Platinum – Applications
Iridium
Iridium is mainly consumed by the automotive, electronic, and chemical industries. Iridium metal is employed when high corrosion resistance at high temperatures is needed, as in high-performance spark plugs, crucibles for recrystallization of semiconductors at high temperatures, and electrodes for the production of chlorine in the chloralkali process. The demand for iridium surged from 2.5 tonnes in 2009 to 10.4 tonnes in 2010, mostly because of electronics-related applications that saw a rise from 0.2 to 6 tonnes – iridium crucibles are commonly used for growing large high-quality single crystals, demand for which has increased sharply.
Platinum
Platinum is primarily an industrial metal. It is a critical material for many industries and is considered a strategic metal. Platinum is used as a catalyst, platinum is mostly found in vehicle catalytic converters that reduce toxic exhaust chemicals, and also in fuel cells to increase efficiency. The most common use of platinum is as a catalyst in chemical reactions, often as platinum black. In catalytic converters, platinum allows the complete combustion of low concentrations of unburned hydrocarbons from the exhaust into carbon dioxide and water vapor. Platinum has been used in thermocouple devices that measure temperature with high accuracy. Platinum is a component in magnetic coatings for high-density hard disk drives and some of the newer optical storage systems.
Iridium and Platinum – Comparison in Table
| Element | Iridium | Platinum |
| Density | 22.65 g/cm3 | 21.09 g/cm3 |
| Ultimate Tensile Strength | 2000 MPa | 150 MPa |
| Yield Strength | N/A | 70 MPa |
| Young’s Modulus of Elasticity | 528 GPa | 168 GPa |
| Mohs Scale | 6.25 | 3.5 |
| Brinell Hardness | 1670 MPa | 400 MPa |
| Vickers Hardness | 1760 MPa | 550 MPa |
| Melting Point | 2410 °C | 1772 °C |
| Boiling Point | 4130 °C | 3827 °C |
| Thermal Conductivity | 150 W/mK | 72 W/mK |
| Thermal Expansion Coefficient | 6.4 µm/mK | 8.8 µm/mK |
| Specific Heat | 0.13 J/g K | 0.13 J/g K |
| Heat of Fusion | 26.1 kJ/mol | 19.6 kJ/mol |
| Heat of Vaporization | 604 kJ/mol | 510 kJ/mol |