About Iridium
Iridium is a very hard, brittle, silvery-white transition metal of the platinum group, iridium is generally credited with being the second densest element (after osmium). It is also the most corrosion-resistant metal, even at temperatures as high as 2000 °C.
Summary
| Element | Iridium |
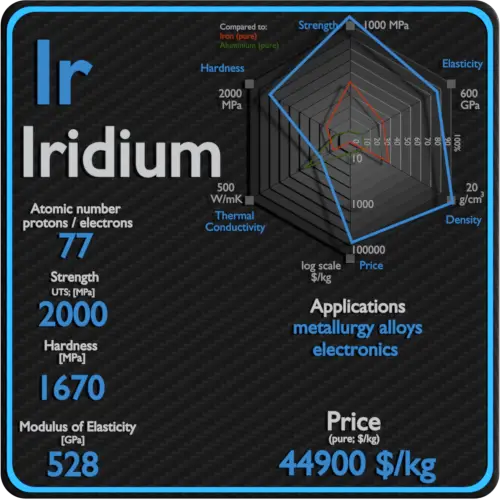
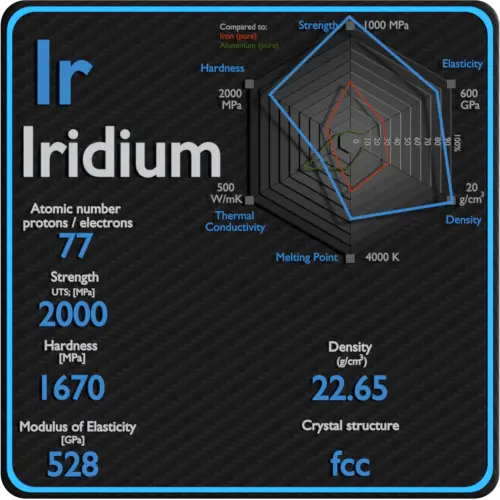
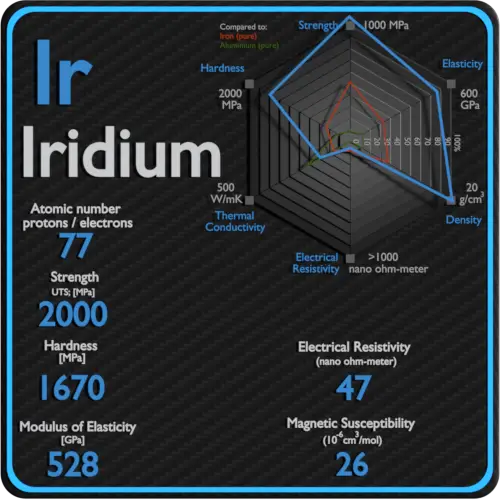
| Atomic number | 77 |
| Element category | Transition Metal |
| Phase at STP | Solid |
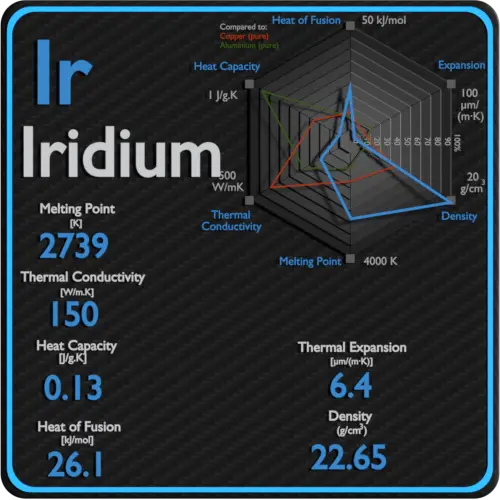
| Density | 22.65 g/cm3 |
| Ultimate Tensile Strength | 2000 MPa |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | 528 GPa |
| Mohs Scale | 6.25 |
| Brinell Hardness | 1670 MPa |
| Vickers Hardness | 1760 MPa |
| Melting Point | 2410 °C |
| Boiling Point | 4130 °C |
| Thermal Conductivity | 150 W/mK |
| Thermal Expansion Coefficient | 6.4 µm/mK |
| Specific Heat | 0.13 J/g K |
| Heat of Fusion | 26.1 kJ/mol |
| Heat of Vaporization | 604 kJ/mol |
| Electrical resistivity [nanoOhm meter] | 47 |
| Magnetic Susceptibility | +26e-6 cm^3/mol |
Applications of Iridium
Iridium is mainly consumed by the automotive, electronic, and chemical industries. Iridium metal is employed when high corrosion resistance at high temperatures is needed, as in high-performance spark plugs, crucibles for recrystallization of semiconductors at high temperatures, and electrodes for the production of chlorine in the chloralkali process. The demand for iridium surged from 2.5 tonnes in 2009 to 10.4 tonnes in 2010, mostly because of electronics-related applications that saw a rise from 0.2 to 6 tonnes – iridium crucibles are commonly used for growing large high-quality single crystals, demand for which has increased sharply.
Production and Price of Iridium
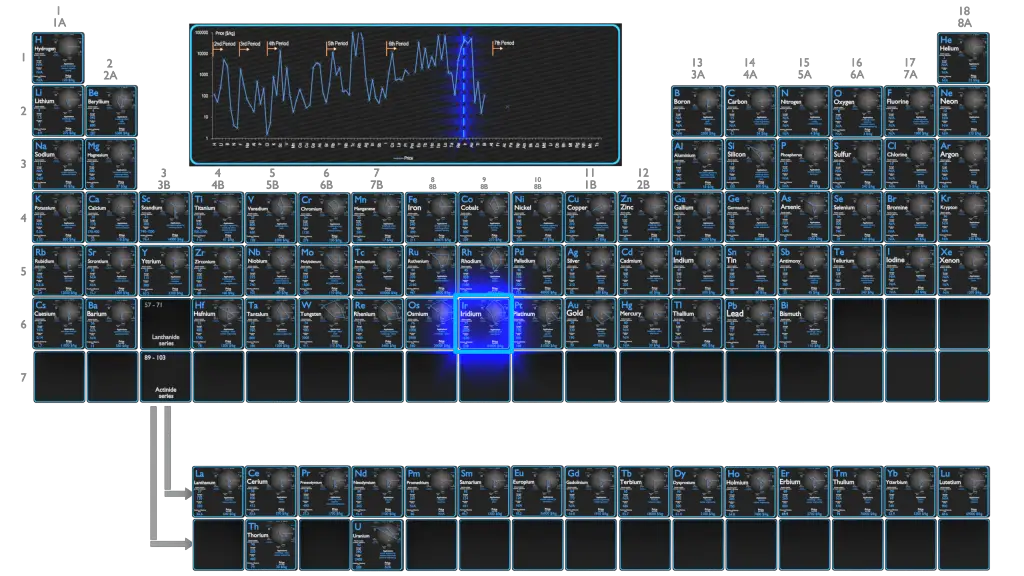
Raw materials prices change daily. They are primarily driven by supply, demand and energy prices. In 2019, prices of pure Iridium were at around 44900 $/kg.
In 2019, worldwide production of iridium totaled 242,000 ounces (6860 kg). Similarly as osmium, iridium concentrates are produced as a by-product of nickel and copper mining or alternatively while isolating the platinum metal from its ores. During electrorefining of copper and nickel, noble metals such as silver, gold and the platinum group metals, together with non-metallic elements such as selenium and tellurium settle to the bottom of the cell as anode mud, which forms the starting material for their extraction.
Source: www.luciteria.com
Mechanical Properties of Iridium
Strength of Iridium
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. In designing structures and machines, it is important to consider these factors, in order that the material selected will have adequate strength to resist applied loads or forces and retain its original shape. Strength of a material is its ability to withstand this applied load without failure or plastic deformation.
For tensile stress, the capacity of a material or structure to withstand loads tending to elongate is known as ultimate tensile strength (UTS). Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins.
See also: Strength of Materials
Ultimate Tensile Strength of Iridium
Ultimate tensile strength of Iridium is 2000 MPa.
Yield Strength of Iridium
Yield strength of Iridium is N/A.
Modulus of Elasticity of Iridium
The Young’s modulus of elasticity of Iridium is N/A.
Hardness of Iridium
In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Brinell hardness test is one of indentation hardness tests, that has been developed for hardness testing. In Brinell tests, a hard, spherical indenter is forced under a specific load into the surface of the metal to be tested.
Brinell hardness of Iridium is approximately 1670 MPa.
The Vickers hardness test method was developed by Robert L. Smith and George E. Sandland at Vickers Ltd as an alternative to the Brinell method to measure the hardness of materials. The Vickers hardness test method can be also used as a microhardness test method, which is mostly used for small parts, thin sections, or case depth work.
Vickers hardness of Iridium is approximately 1760 MPa.
Scratch hardness is the measure of how resistant a sample is to permanent plastic deformation due to friction from a sharp object. The most common scale for this qualitative test is Mohs scale, which is used in mineralogy. The Mohs scale of mineral hardness is based on the ability of one natural sample of mineral to scratch another mineral visibly.
Iridium is has a hardness of approximately 6.25.
See also: Hardness of Materials
Iridium – Crystal Structure
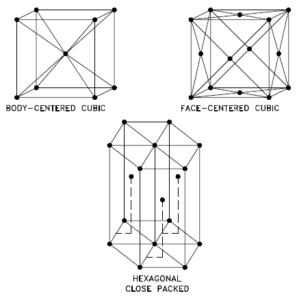
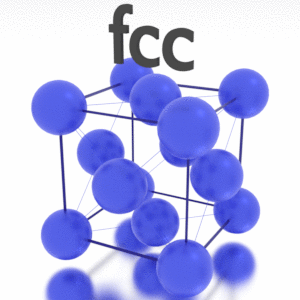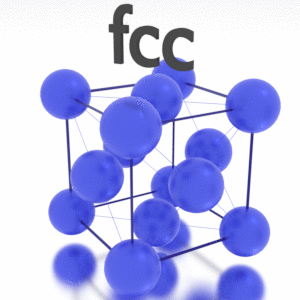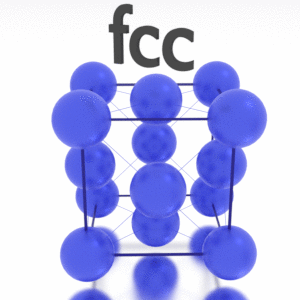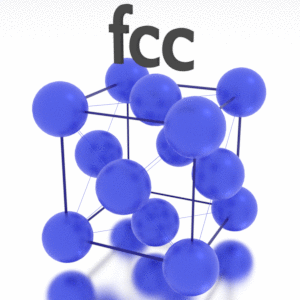
A possible crystal structure of Iridium is face-centered cubic structure.
In metals, and in many other solids, the atoms are arranged in regular arrays called crystals. A crystal lattice is a repeating pattern of mathematical points that extends throughout space. The forces of chemical bonding causes this repetition. It is this repeated pattern which control properties like strength, ductility, density, conductivity (property of conducting or transmitting heat, electricity, etc.), and shape. There are 14 general types of such patterns known as Bravais lattices.
See also: Crystal Structure of Materials
Crystal Structure of Iridium
Thermal Properties of Iridium
Iridium – Melting Point and Boiling Point
Melting point of Iridium is 2410°C.
Boiling point of Iridium is 4130°C.
Note that, these points are associated with the standard atmospheric pressure.
Iridium – Thermal Conductivity
Thermal conductivity of Iridium is 150 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
Coefficient of Thermal Expansion of Iridium
Linear thermal expansion coefficient of Iridium is 6.4 µm/(m·K)
Thermal expansion is generally the tendency of matter to change its dimensions in response to a change in temperature. It is usually expressed as a fractional change in length or volume per unit temperature change.
Iridium – Specific Heat, Latent Heat of Fusion, Latent Heat of Vaporization
Specific heat of Iridium is 0.13 J/g K.
Heat capacity is an extensive property of matter, meaning it is proportional to the size of the system. Heat capacity C has the unit of energy per degree or energy per kelvin. When expressing the same phenomenon as an intensive property, the heat capacity is divided by the amount of substance, mass, or volume, thus the quantity is independent of the size or extent of the sample.
Latent Heat of Fusion of Iridium is 26.1 kJ/mol.
Latent Heat of Vaporization of Iridium is 604 kJ/mol.
Latent heat is the amount of heat added to or removed from a substance to produce a change in phase. This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the gas (the pΔV work). When latent heat is added, no temperature change occurs. The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
Iridium – Electrical Resistivity – Magnetic Susceptibility
Electrical property refers to the response of a material to an applied electric field. One of the principal characteristics of materials is their ability (or lack of ability) to conduct electrical current. Indeed, materials are classified by this property, that is, they are divided into conductors, semiconductors, and nonconductors.
See also: Electrical Properties
Magnetic property refers to the response of a material to an applied magnetic field. The macroscopic magnetic properties of a material are a consequence of interactions between an external magnetic field and the magnetic dipole moments of the constituent atoms. Different materials react to the application of magnetic field differently.
See also: Magnetic Properties
Electrical Resistivity of Iridium
Electrical resistivity of Iridium is 47 nΩ⋅m.
Electrical conductivity and its converse, electrical resistivity, is a fundamental property of a material that quantifies how Iridium conducts the flow of electric current. Electrical conductivity or specific conductance is the reciprocal of electrical resistivity.
Magnetic Susceptibility of Iridium
Magnetic susceptibility of Iridium is +26e-6 cm^3/mol.
In electromagnetism, magnetic susceptibility is the measure of the magnetization of a substance. Magnetic susceptibility is a dimensionless proportionality factor that indicates the degree of magnetization of Iridium in response to an applied magnetic field.