This article contains comparison of key thermal and atomic properties of aluminium and iron, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Aluminium vs Iron.
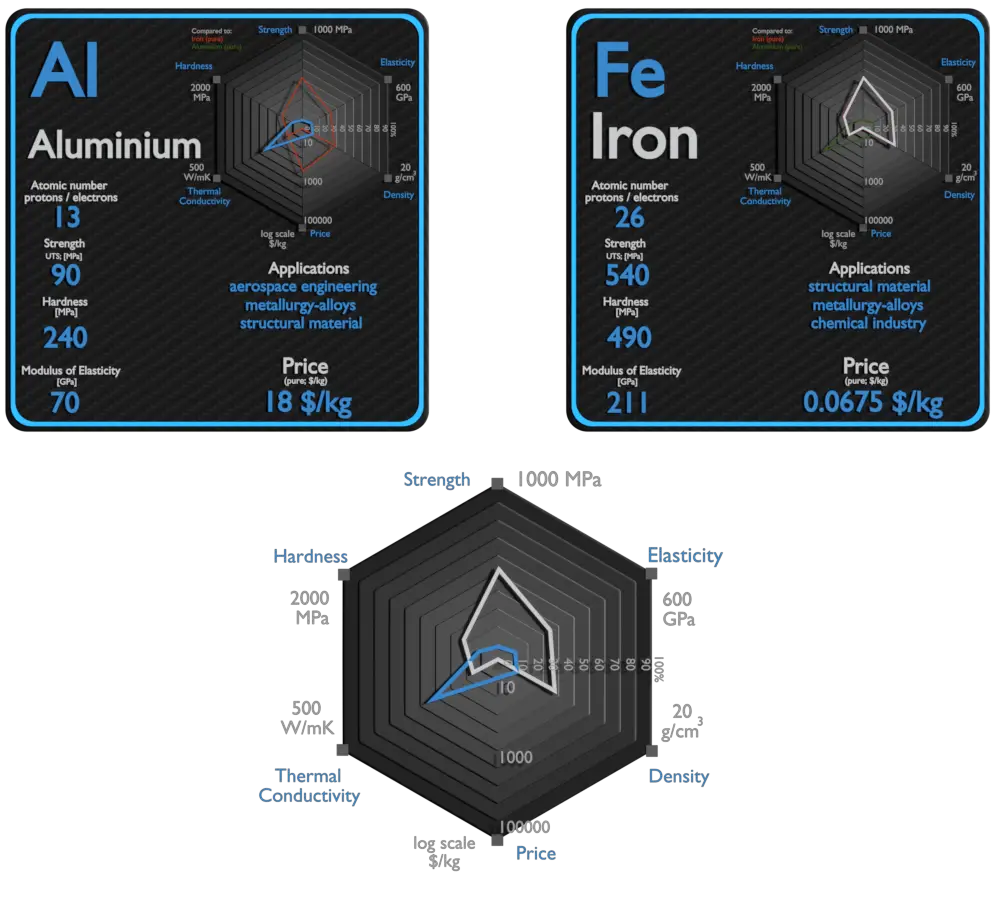
Aluminium and Iron – About Elements


Source: www.luciteria.com
Aluminium and Iron – Applications
Aluminium
Aluminium and its alloys are used widely in aerospace, automotive, architectural, lithographic, packaging, electrical and electronic applications. It is the prime material of construction for the aircraft industry throughout most of its history. About 70% of commercial civil aircraft airframes are made from aluminium alloys, and without aluminium civil aviation would not be economically viable. Automotive industry now includes aluminium as engine castings, wheels, radiators and increasingly as body parts. 6111 aluminium and 2008 aluminium alloy are extensively used for external automotive body panels. Cylinder blocks and crankcases are often cast made of aluminium alloys.
Iron
Iron is used in numerous sectors such as electronics, manufacturing, automotive, and construction and building. Iron is the most widely used of all the metals, accounting for over 90% of worldwide metal produc0tion. Its low cost and high strength often make it the material of choice material to withstand stress or transmit forces, such as the construction of machinery and machine tools, rails, automobiles, ship hulls, concrete reinforcing bars, and the load-carrying framework of buildings. Since pure iron is quite soft, it is most commonly combined with alloying elements to make steel. Steels are iron–carbon alloys that may contain appreciable concentrations of other alloying elements. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Due to its very-high strength, but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloy in modern use. There are thousands of alloys that have different compositions and/or heat treatments. The mechanical properties are sensitive to the content of carbon, which is normally less than 1.0 wt%.
Aluminium and Iron – Comparison in Table
| Element | Aluminium | Iron |
| Density | 2.7 g/cm3 | 7.874 g/cm3 |
| Ultimate Tensile Strength | 90 MPa (pure), 600 MPa (alloys) | 540 MPa |
| Yield Strength | 11 MPa (pure), 400 MPa (alloys) | 50 MPa |
| Young’s Modulus of Elasticity | 70 GPa | 211 GPa |
| Mohs Scale | 2.8 | 4.5 |
| Brinell Hardness | 240 MPa | 490 MPa |
| Vickers Hardness | 167 MPa | 608 MPa |
| Melting Point | 660 °C | 1538 °C |
| Boiling Point | 2467 °C | 2861 °C |
| Thermal Conductivity | 237 W/mK | 80.2 W/mK |
| Thermal Expansion Coefficient | 23.1 µm/mK | 11.8 µm/mK |
| Specific Heat | 0.9 J/g K | 0.44 J/g K |
| Heat of Fusion | 10.79 kJ/mol | 13.8 kJ/mol |
| Heat of Vaporization | 293.4 kJ/mol | 349.6 kJ/mol |