This article contains comparison of key thermal and atomic properties of magnesium and aluminium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Magnesium vs Aluminium.
Magnesium and Aluminium – About Elements


Source: www.luciteria.com
Magnesium and Aluminium – Applications
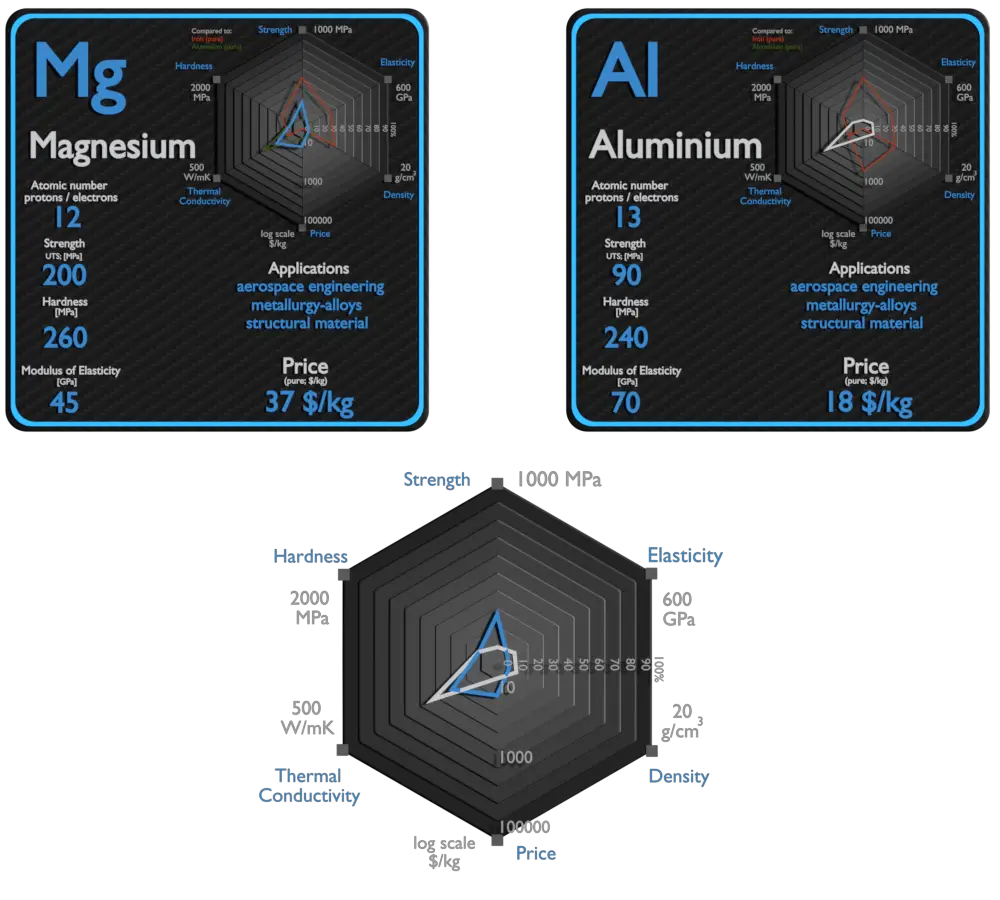
Magnesium
Magnesium is the third-most-commonly-used structural metal, following iron and aluminium.[35] The main applications of magnesium are, in order: aluminium alloys, die-casting (alloyed with zinc), removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process. Magnesium alloys are used in a wide variety of structural and nonstructural applications. Structural applications include automotive, industrial, materials-handling, commercial, and aerospace equipment. Magnesium alloys are used for parts that operate at high speeds and thus must be light weight to minimize inertial forces. Commercial applications include hand-held tools, laptops, luggage, and ladders, automobiles (e.g., steering wheels and columns, seat frames, transmission cases). Magnox (alloy), whose name is an abbreviation for “magnesium non-oxidizing”, is 99% magnesium and 1% aluminum, and is used in the cladding of fuel rods in magnox nuclear power reactors.
Aluminium
Aluminium and its alloys are used widely in aerospace, automotive, architectural, lithographic, packaging, electrical and electronic applications. It is the prime material of construction for the aircraft industry throughout most of its history. About 70% of commercial civil aircraft airframes are made from aluminium alloys, and without aluminium civil aviation would not be economically viable. Automotive industry now includes aluminium as engine castings, wheels, radiators and increasingly as body parts. 6111 aluminium and 2008 aluminium alloy are extensively used for external automotive body panels. Cylinder blocks and crankcases are often cast made of aluminium alloys.
Magnesium and Aluminium – Comparison in Table
| Element | Magnesium | Aluminium |
| Density | 1.738 g/cm3 | 2.7 g/cm3 |
| Ultimate Tensile Strength | 200 MPa | 90 MPa (pure), 600 MPa (alloys) |
| Yield Strength | N/A | 11 MPa (pure), 400 MPa (alloys) |
| Young’s Modulus of Elasticity | 45 GPa | 70 GPa |
| Mohs Scale | 2.5 | 2.8 |
| Brinell Hardness | 260 MPa | 240 MPa |
| Vickers Hardness | N/A | 167 MPa |
| Melting Point | 649 °C | 660 °C |
| Boiling Point | 1090 °C | 2467 °C |
| Thermal Conductivity | 156 W/mK | 237 W/mK |
| Thermal Expansion Coefficient | 24.8 µm/mK | 23.1 µm/mK |
| Specific Heat | 1.02 J/g K | 0.9 J/g K |
| Heat of Fusion | 8.954 kJ/mol | 10.79 kJ/mol |
| Heat of Vaporization | 127.4 kJ/mol | 293.4 kJ/mol |