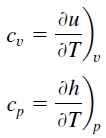About Samarium
Samarium is a typical member of the lanthanide series, it is a moderately hard silvery metal that readily oxidizes in air. The name samarium is after the mineral samarskite from which it was isolated. Although classified as a rare earth element, samarium is the 40th most abundant element in the Earth’s crust and is more common than such metals as tin. In nuclear industry, especially natural and artificial samarium 149 has an important impact on the operation of a nuclear reactor. Samarium 149 has a very large neutron capture cross-section (about 42,000 barns). Since natural samarium contains about 14% of 149Sm, it can be used as an absorbing material in control rods.
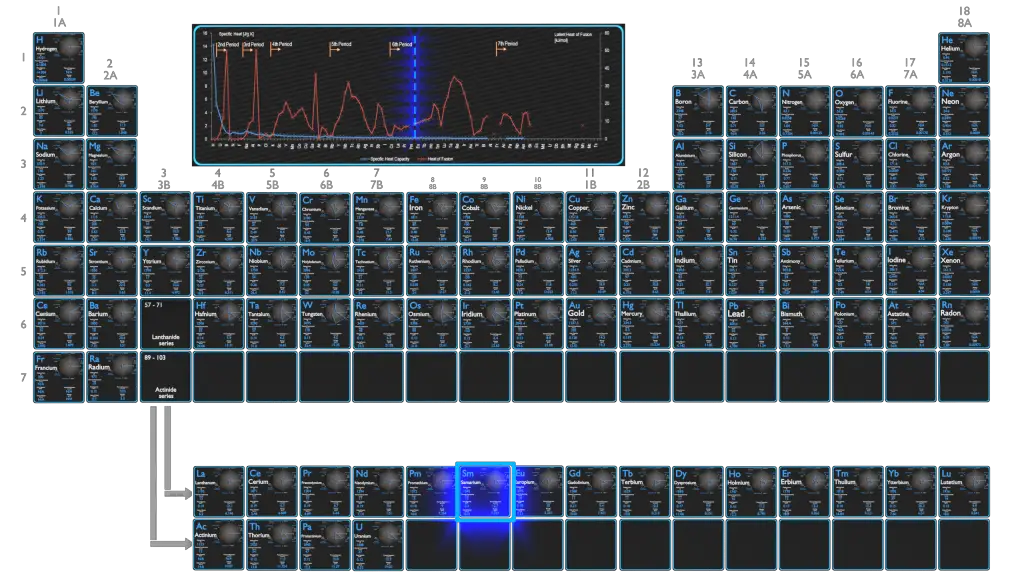
Samarium – Specific Heat, Latent Heat of Fusion, Latent Heat of Vaporization
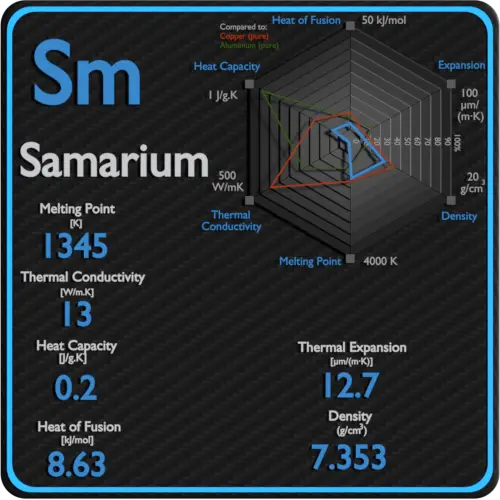
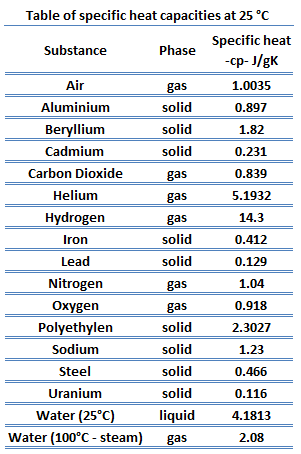
Specific heat of Samarium is 0.2 J/g K.
Heat capacity is an extensive property of matter, meaning it is proportional to the size of the system. Heat capacity C has the unit of energy per degree or energy per kelvin. When expressing the same phenomenon as an intensive property, the heat capacity is divided by the amount of substance, mass, or volume, thus the quantity is independent of the size or extent of the sample.
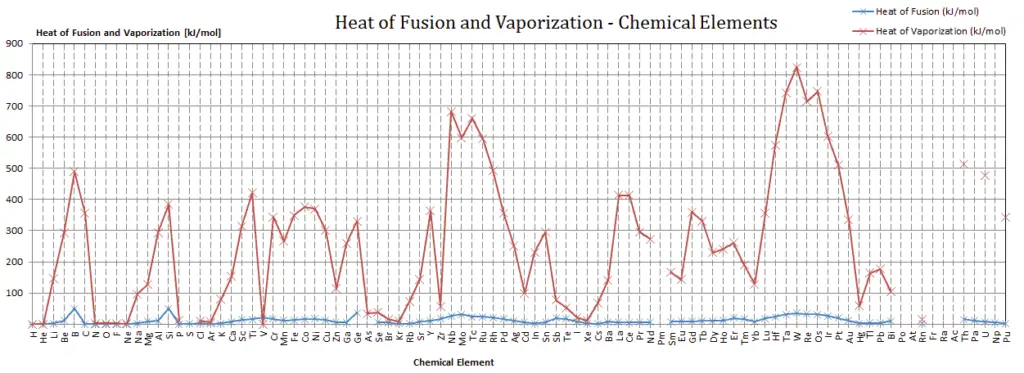
Latent Heat of Fusion of Samarium is 8.63 kJ/mol.
Latent Heat of Vaporization of Samarium is 192 kJ/mol.
Latent heat is the amount of heat added to or removed from a substance to produce a change in phase. This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the gas (the pΔV work). When latent heat is added, no temperature change occurs. The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
See also: Mechanical Properties of Samarium
Summary
| Element | Samarium |
| Specific Heat | 0.2 J/g K |
| Heat of Fusion | 8.63 kJ/mol |
| Heat of Vaporization | 192 kJ/mol |
| Density | 7.353 g/cm3 |
Source: www.luciteria.com