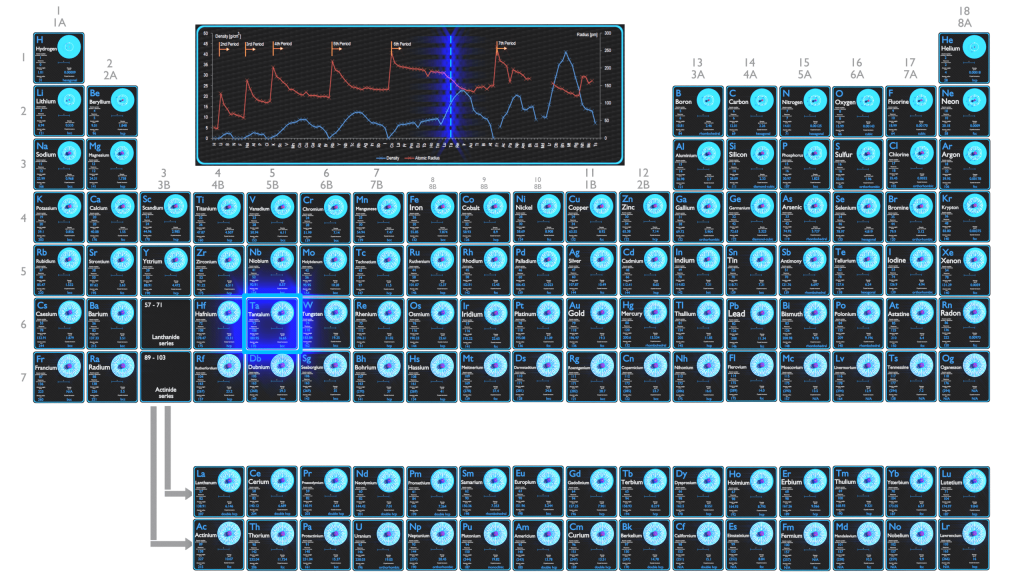
Tantalum is a rare, hard, blue-gray, shiny transition metal that is highly resistant to corrosion.
Resumen
| Element | Tantalum |
| Atomic number | 73 |
| Atomic mass [amu] | 180.9479 |
| Atomic mass [pm] | 170 |
| Density at STP [g / cm3] | 16.65 |
| Number of protons | 73 |
| Number of neutrons (typical isotopes) | 181 |
| Number of electrons | 73 |
| Electron configuration | [Xe] 4f14 5d3 6s2 |
| Oxidation states | +5 |
| Electron affinity [kJ/mol] | 31 |
| Electronegativity [Pauling scale] | 1.5 |
| First ionization energy [eV] | 7.89 |
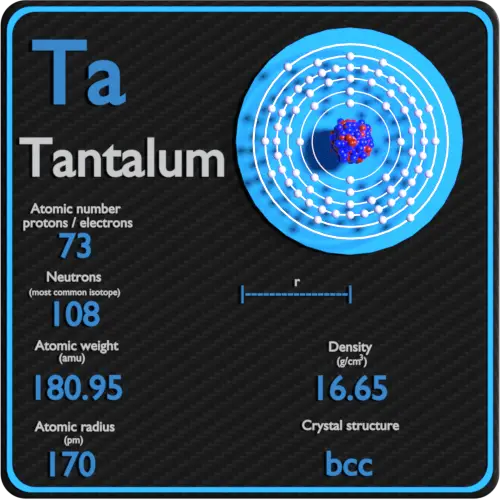
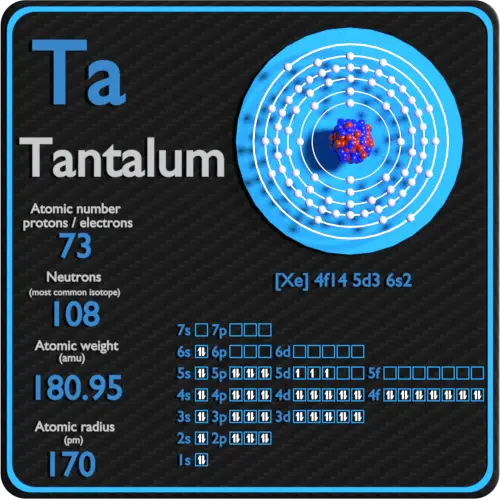
Atomic number: protons, electrons and neutrons in Tantalum
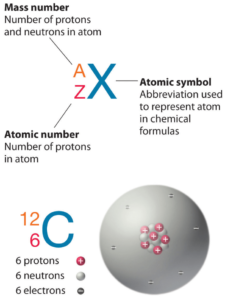
Tantalum is a chemical element with atomic number 73, which means that there are 73 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electric charge of the nucleus is therefore + Ze, where e (elemental charge) is equal to 1.602 x 10-19 coulombs.
The total number of neutrons in the nucleus of an atom is called the number of neutrons in the atom and is given the symbol N. Number of neutrons plus atomic number equals atomic mass number: N + Z = A. The difference between the number of neutrons and the atomic number is known as the excess neutrons: D = N – Z = A – 2Z.
For stable elements, there are usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The typical isotope mass of tantalum is 181.
Atomic mass of Tantalum
The atomic mass of tantalum is 180.9479 u.
Atomic mass is the mass of an atom. Atomic mass or relative isotopic mass refers to the mass of a single particle and is therefore bound to a certain specific isotope of an element. Atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but contains all of the positive charge and at least 99.95% of the total mass of the atom. Note that each element can contain more isotopes, therefore this resulting atomic mass is calculated from the natural isotopes and their abundance.
Atomic radius of Tantalum
The atomic radius of the tantalum atom is 170 pm (covalent radius).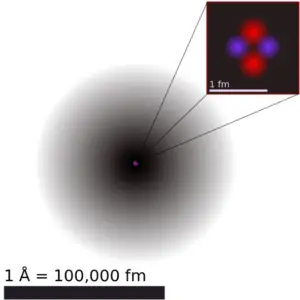
It should be noted that atoms lack a well-defined outer limit. The atomic radius of a chemical element is a measure of the distance the electron cloud extends from the nucleus. However, this assumes that the atom exhibits a spherical shape, which is only obeyed for atoms in a vacuum or free space. Therefore, there are several non-equivalent definitions of atomic radius.
Electrons and electron configuration
The number of electrons in an electrically neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in the neutral tantalum atom is 73. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other negative electrons (Z – 1) in the atom.
Since the number of electrons and their arrangement are responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. The configuration of these electrons is derived from the principles of quantum mechanics. The number of electrons in the electron shells of each element, particularly the outermost valence shell, is the main factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
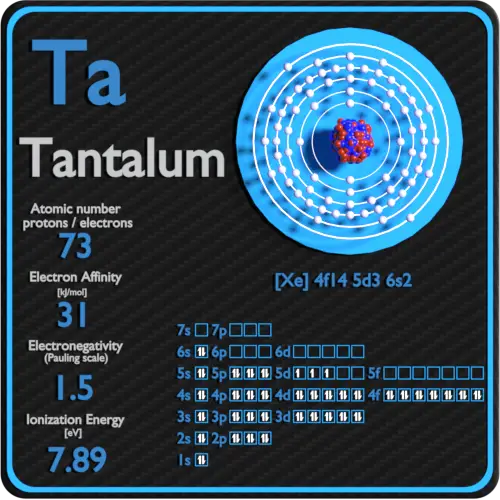
The electron configuration of tantalum is [Xe] 4f14 5d3 6s2.
Possible oxidation states are +5.
Tantalum density
The density of tantalum is 16.65 g / cm 3 .
Typical densities of various substances are at atmospheric pressure.
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
Electron Affinity – Tantalum
Electron affinity of Tantalum is 31 kJ/mol.
In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:
the change in energy (in kJ/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.
X + e– → X– + energy Affinity = – ∆H
In other words, it can be expressed as the neutral atom’s likelihood of gaining an electron. Note that, ionization energies measure the tendency of a neutral atom to resist the loss of electrons. Electron affinities are more difficult to measure than ionization energies.
Electronegativity of Tantalum
Electronegativity of Tantalum is 1.5.
Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. For this purposes, a dimensionless quantity the Pauling scale, symbol χ, is the most commonly used.
The electronegativity of Tantalum is: χ = 1.5
First Ionization Energy of Tantalum
First Ionization Energy of Tantalum is 7.89 eV.
Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.
X + energy → X+ + e−
where X is any atom or molecule capable of being ionized, X+ is that atom or molecule with an electron removed (positive ion), and e− is the removed electron.
A Tantalum atom, for example, requires the following ionization energy to remove the outermost electron.
Ta + IE → Ta+ + e− IE = 7.89 eV
Source: www.luciteria.com