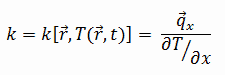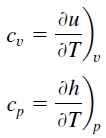About Methane
Methane, CH4, is a colorless odorless gas. It is also known as marsh gas or methyl hydride. The vapors are lighter than air. It is a group-14 hydride and the simplest alkane, and is the main constituent of natural gas.
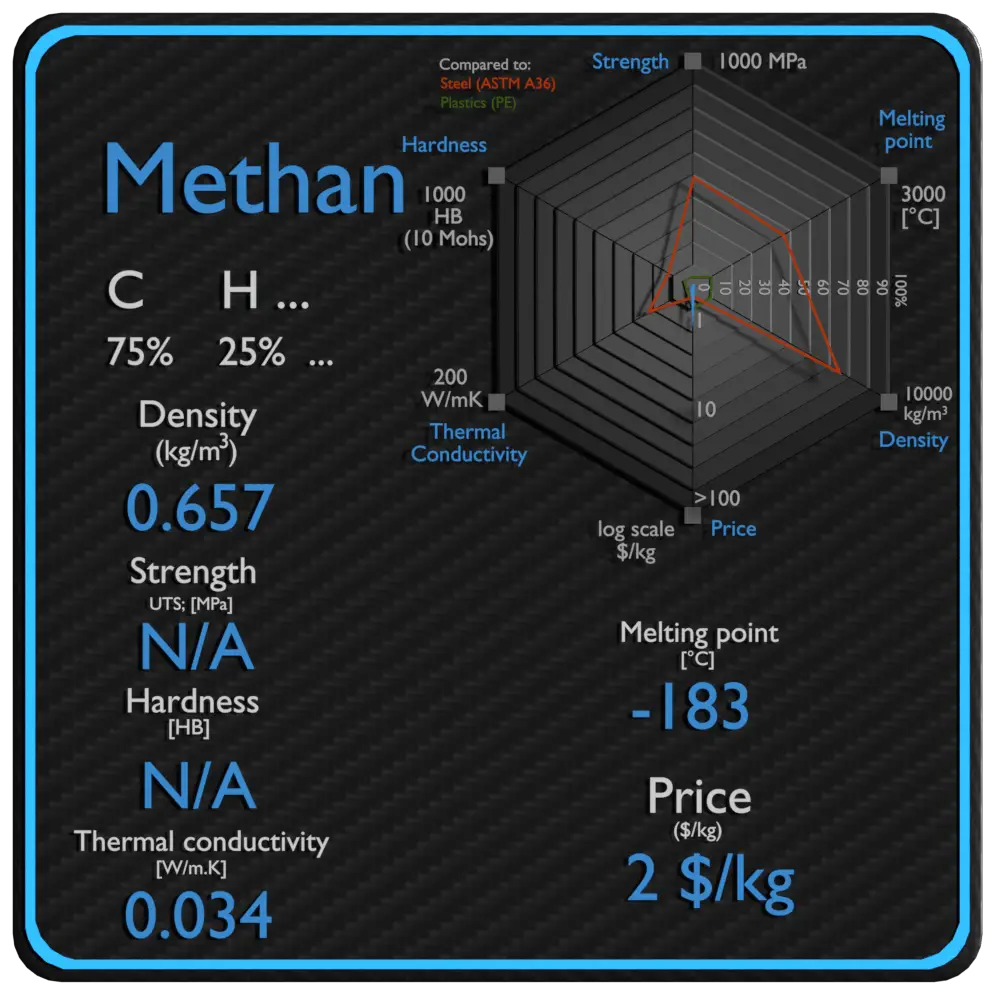
Summary
| Name | Methane |
| Phase at STP | gas |
| Density | 0.657 kg/m3 |
| Ultimate Tensile Strength | N/A |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | N/A |
| Brinell Hardness | N/A |
| Melting Point | -183 °C |
| Thermal Conductivity | 0.034 W/mK |
| Heat Capacity | 2200 J/g K |
| Price | 2 $/kg |
Composition of Methane
Methane is a chemical compound with the chemical formula CH4 (one atom of carbon and four atoms of hydrogen).
Applications of Methane
Methane is a fuel and can be used in industrial chemical processes and may be transported as a refrigerated liquid (liquefied natural gas, or LNG). Methane is used as a fuel for ovens, homes, water heaters, kilns, automobiles, turbines, and other things. Natural gas is a naturally occurring hydrocarbon gas mixture consisting primarily of methane, but commonly including varying amounts of other higher alkanes.
Thermal Properties of Methane
Methane – Melting Point
Melting point of Methane is -183 °C.
Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. For various chemical compounds and alloys, it is difficult to define the melting point, since they are usually a mixture of various chemical elements.
Methane – Thermal Conductivity
Thermal conductivity of Methane is 0.034 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Methane – Specific Heat
Specific heat of Methane is 2200 J/g K.
Specific heat, or specific heat capacity, is a property related to internal energy that is very important in thermodynamics. The intensive properties cv and cp are defined for pure, simple compressible substances as partial derivatives of the internal energy u(T, v) and enthalpy h(T, p), respectively:
where the subscripts v and p denote the variables held fixed during differentiation. The properties cv and cp are referred to as specific heats (or heat capacities) because under certain special conditions they relate the temperature change of a system to the amount of energy added by heat transfer. Their SI units are J/kg K or J/mol K.
Properties and prices of other materials
material-table-in-8k-resolution