This article contains comparison of key thermal and atomic properties of zirconium and uranium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Zirconium vs Uranium.
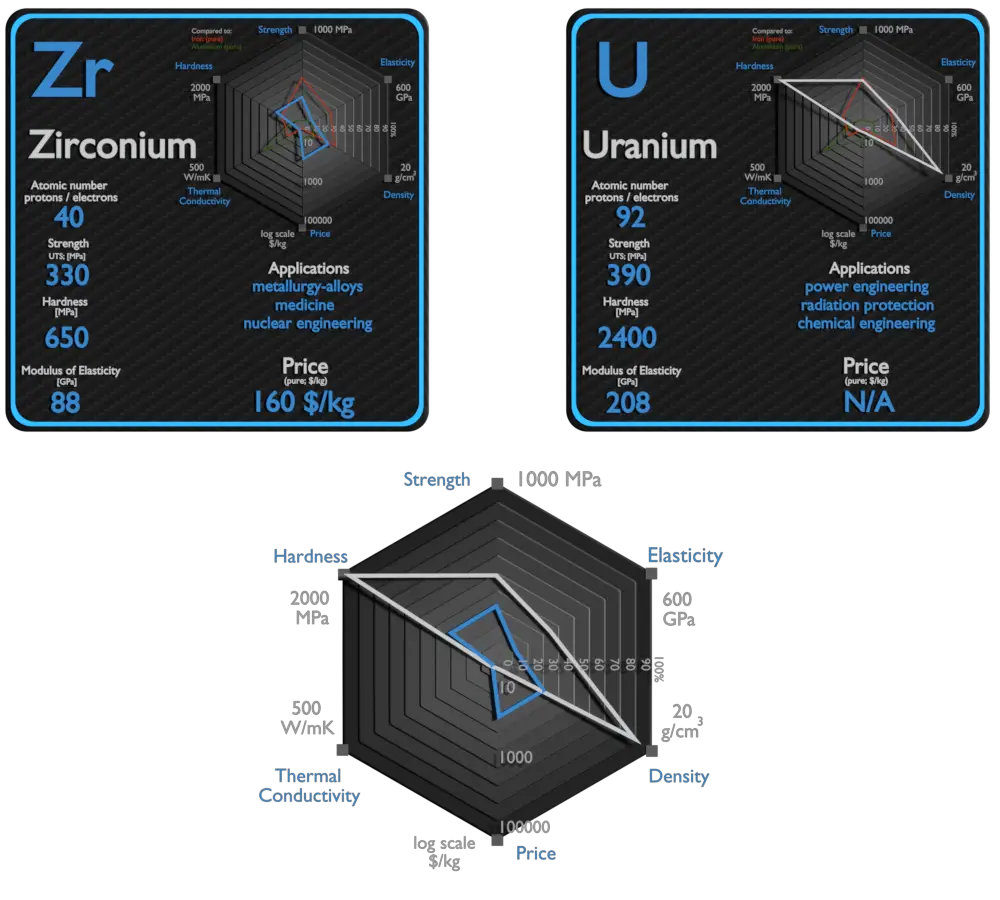
Zirconium and Uranium – About Elements


Source: www.luciteria.com
Zirconium and Uranium – Applications
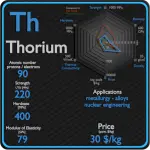
Zirconium
Most zircon is used directly in high-temperature applications. This material is refractory, hard, and resistant to chemical attack. Because of these properties, zircon finds many applications, few of which are highly publicized. Its main use is as an opacifier, conferring a white, opaque appearance to ceramic materials. Zirconium and its alloys are widely used as a cladding for nuclear reactor fuels. Zirconium alloyed with niobium or tin has excellent corrosion properties. The high corrosion resistance of zirconium alloys results from the natural formation of a dense stable oxide on the surface of the metal. This film is self healing, it continues to grow slowly at temperatures up to approximately 550 °C (1020 °F), and it remains tightly adherent. The desired property of these alloys is also a low neutron-capture cross-section. The disadvantages of zirconium are low strength properties and low heat resistance, which can be eliminated, for example, by alloying with niobium.
Uranium
The main use of uranium in the civilian sector is to fuel nuclear power plants. One kilogram of uranium-235 can theoretically produce about 20 terajoules of energy, assuming complete fission; as much energy as 1.5 million kilograms (1,500 tonnes) of coal. Typical reactor may contain about 100 tonnes of enriched uranium (i.e. about 113 tonnes of uranium dioxide). This fuel is loaded within, for example, 157 fuel assemblies composed of over 45,000 fuel rods. A common fuel assembly contain energy for approximately 4 years of operation at full power. The removed fuel (spent nuclear fuel) still contains about 96% of reusable material (it must be removed due to decreasing kinf of an assembly). Before (and, occasionally, after) the discovery of radioactivity, uranium was primarily used in small amounts for yellow glass and pottery glazes, such as uranium glass. Uranium is also used by the military to power nuclear submarines and in nuclear weapons. Due to its high density, this material is found in inertial guidance systems and in gyroscopic compasses.[10] Depleted uranium is preferred over similarly dense metals due to its ability to be easily machined and cast as well as its relatively low cost. The main risk of exposure to depleted uranium is chemical poisoning by uranium oxide rather than radioactivity (uranium being only a weak alpha emitter). Depleted uranium is uranium that has much less uranium-235 than natural uranium. It is considerably less radioactive than natural uranium. It is a dense metal that can be used as ballast for ships and counterweights for aircraft. It is also used in ammunition and armour. Depleted uranium can be also used to shield radiation. Depleted uranium is much more effective due to its higher Z. Depleted uranium is used for shielding in portable gamma ray sources. Uranium is used in high-speed steels as an alloying agent to improve strength and toughness. Uranium trioxide (also called uranic oxide) with formula UO3, is an orange-yellow powder and is used as a pigment for ceramics. In glasses it produces a beautiful greenish-yellow “uranium glass”.
Zirconium and Uranium – Comparison in Table
| Element | Zirconium | Uranium |
| Density | 6.511 g/cm3 | 19.05 g/cm3 |
| Ultimate Tensile Strength | 330 MPa | 390 MPa |
| Yield Strength | 230 MPa | 190 MPa |
| Young’s Modulus of Elasticity | 88 GPa | 208 GPa |
| Mohs Scale | 5 | 6 |
| Brinell Hardness | 650 MPa | 2400 MPa |
| Vickers Hardness | 900 MPa | 1960 MPa |
| Melting Point | 1855 °C | 1132 °C |
| Boiling Point | 4377 °C | 4131 °C |
| Thermal Conductivity | 22.7 W/mK | 27 W/mK |
| Thermal Expansion Coefficient | 5.7 µm/mK | 13.9 µm/mK |
| Specific Heat | 0.27 J/g K | 0.12 J/g K |
| Heat of Fusion | 16.9 kJ/mol | 8.52 kJ/mol |
| Heat of Vaporization | 591 kJ/mol | 417 kJ/mol |