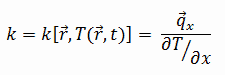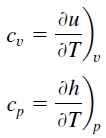About Acetylene
Acetylene is a colorless, highly flammable gas with an ethereal odor. Acetylene is a hydrocarbon and the simplest alkyne with the formula C2H2. Commercial acetylene will have a garlic-like odor. It is shipped with dissolved acetone in its gaseous form. Typical uses oxyacetylene welding and chemical synthesis.
Summary
| Name | Acetylene |
| Phase at STP | gaseous |
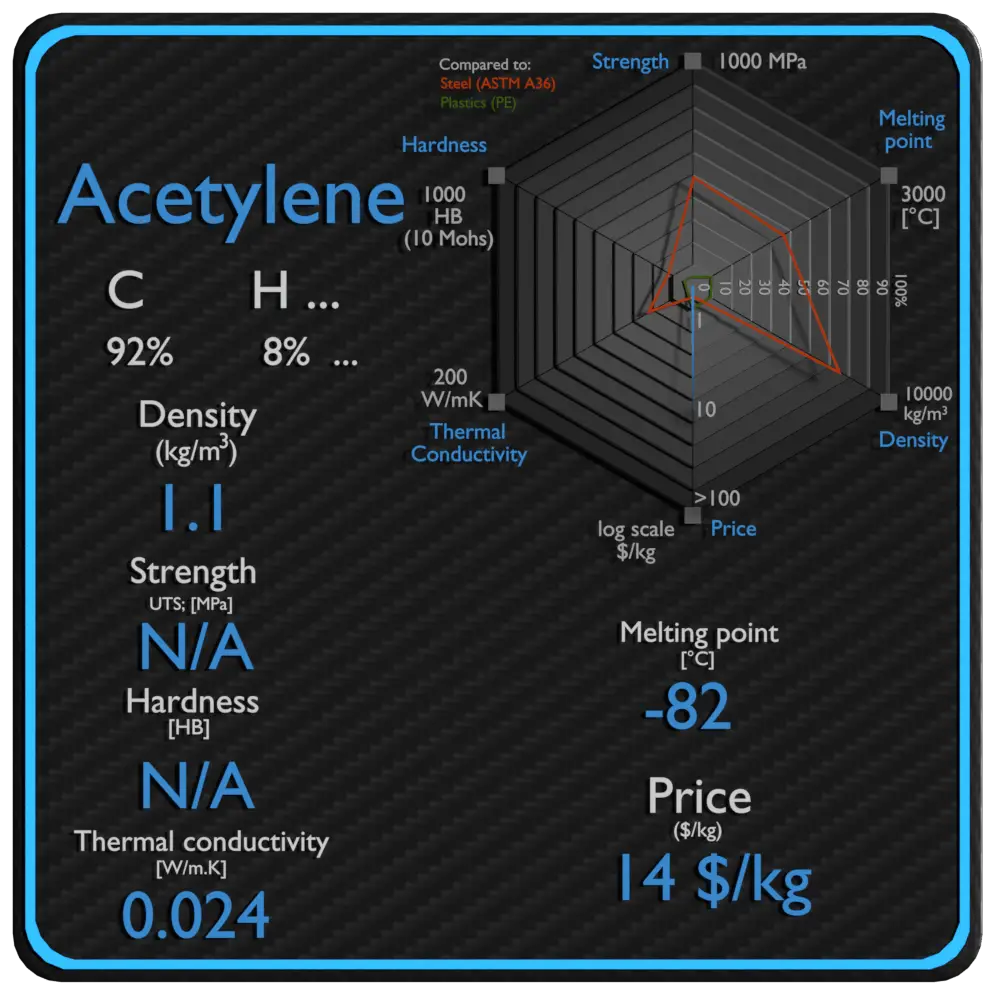
| Density | 1.1 kg/m3 |
| Ultimate Tensile Strength | N/A |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | N/A |
| Brinell Hardness | N/A |
| Melting Point | -82 °C |
| Thermal Conductivity | 0.024 W/mK |
| Heat Capacity | 1674 J/g K |
| Price | 14 $/kg |
Composition of Acetylene
Acetylene is a hydrocarbon and the simplest alkyne with the formula C2H2. As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°.
Applications of Acetylene
Typical uses oxyacetylene chemical synthesis and especially for welding and cutting. Among all other gases, acetylene is capable of producing the hottest flame. The welding process that uses acetylene is known as oxy-fuel cutting or gas cutting. This method is used to cut or weld materials that require temperatures as high as 3,500 °C.
Thermal Properties of Acetylene
Acetylene – Melting Point
Melting point of Acetylene is -82 °C.
Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. For various chemical compounds and alloys, it is difficult to define the melting point, since they are usually a mixture of various chemical elements.
Acetylene – Thermal Conductivity
Thermal conductivity of Acetylene is 0.024 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Acetylene – Specific Heat
Specific heat of Acetylene is 1674 J/g K.
Specific heat, or specific heat capacity, is a property related to internal energy that is very important in thermodynamics. The intensive properties cv and cp are defined for pure, simple compressible substances as partial derivatives of the internal energy u(T, v) and enthalpy h(T, p), respectively:
where the subscripts v and p denote the variables held fixed during differentiation. The properties cv and cp are referred to as specific heats (or heat capacities) because under certain special conditions they relate the temperature change of a system to the amount of energy added by heat transfer. Their SI units are J/kg K or J/mol K.
Properties and prices of other materials
material-table-in-8k-resolution