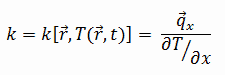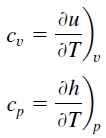About Diesel Fuel
In general, diesel fuel is any liquid fuel specifically designed for use in diesel engines, whose fuel ignition takes place, without any spark, as a result of compression of the inlet air mixture and then injection of fuel. Currently, the main source of fuel for road vehicles is petroleum oil. Oil is a naturally occurring, fossil liquid comprising a complex mixture of hydrocarbons of various molecular weights together with various other organic compounds. These components are separated by fractional distillation in an oil refinery in order to produce gasoline, diesel fuel, kerosene and other products.
Summary
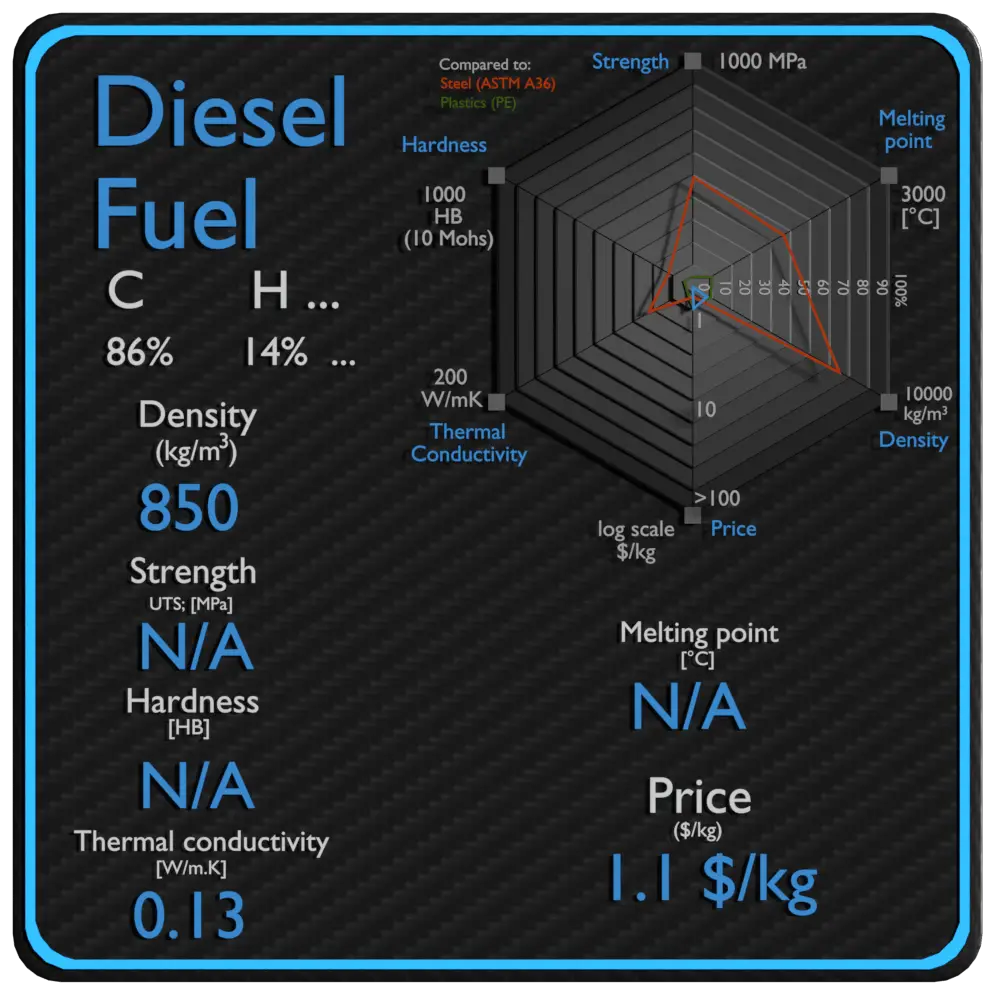
| Name | Diesel Fuel |
| Phase at STP | liquid |
| Density | 850 kg/m3 |
| Ultimate Tensile Strength | N/A |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | N/A |
| Brinell Hardness | N/A |
| Melting Point | N/A |
| Thermal Conductivity | 0.13 W/mK |
| Heat Capacity | 2100 J/g K |
| Price | 1.1 $/kg |
Composition of Diesel Fuel
Diesel fuel is used primarily as a fuel in most diesel engines. Diesel fuel is mostly used in high-speed diesel engines, especially motor-vehicle (e.g. car, lorry) diesel engines, but not all diesel engines run on diesel fuel. The usage and pricing of gasoline (or petrol) results from factors such as crude oil prices, processing and distribution costs, local demand, the strength of local currencies, local taxation, and the availability of local sources of gasoline (supply). Since fuels are traded worldwide, the trade prices are similar. The price paid by consumers largely reflects national pricing policy. Some regions, such as Europe and Japan, impose high taxes on gasoline (petrol); others, such as Saudi Arabia and Venezuela, subsidize the cost.
Applications of Diesel Fuel

Diesel, or diesel fuel, refers in general to any fuel that can be used in a diesel engine. Petroleumderived diesel (PDD) is composed of ca. 64-75% saturated hydrocarbons (primarily paraffins including n-, iso-, and cyclo-paraffins), l-2% olefinic hydrocarbons and 25-35% aromatic hydrocarbons (including naphthalenes and alkylbenzenes). Although the average chemical formula for common diesel fuel is C12H23, components range from approximately C10H20 to C15H28.
Thermal Properties of Diesel Fuel
Diesel Fuel – Melting Point
Melting point of Diesel Fuel is N/A.
Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. For various chemical compounds and alloys, it is difficult to define the melting point, since they are usually a mixture of various chemical elements.
Diesel Fuel – Thermal Conductivity
Thermal conductivity of Diesel Fuel is 0.13 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Diesel Fuel – Specific Heat
Specific heat of Diesel Fuel is 2100 J/g K.
Specific heat, or specific heat capacity, is a property related to internal energy that is very important in thermodynamics. The intensive properties cv and cp are defined for pure, simple compressible substances as partial derivatives of the internal energy u(T, v) and enthalpy h(T, p), respectively:
where the subscripts v and p denote the variables held fixed during differentiation. The properties cv and cp are referred to as specific heats (or heat capacities) because under certain special conditions they relate the temperature change of a system to the amount of energy added by heat transfer. Their SI units are J/kg K or J/mol K.
Properties and prices of other materials
material-table-in-8k-resolution