About Air
Air is a mixture of nitrogen, oxygen, argon, carbon dioxide, water vapour, and other trace elements. The atmosphere of Earth is the layer of gases, commonly known as air, retained by Earth’s gravity, surrounding the planet Earth and forming its planetary atmosphere.
Summary
| Name | Air |
| Phase at STP | gas |
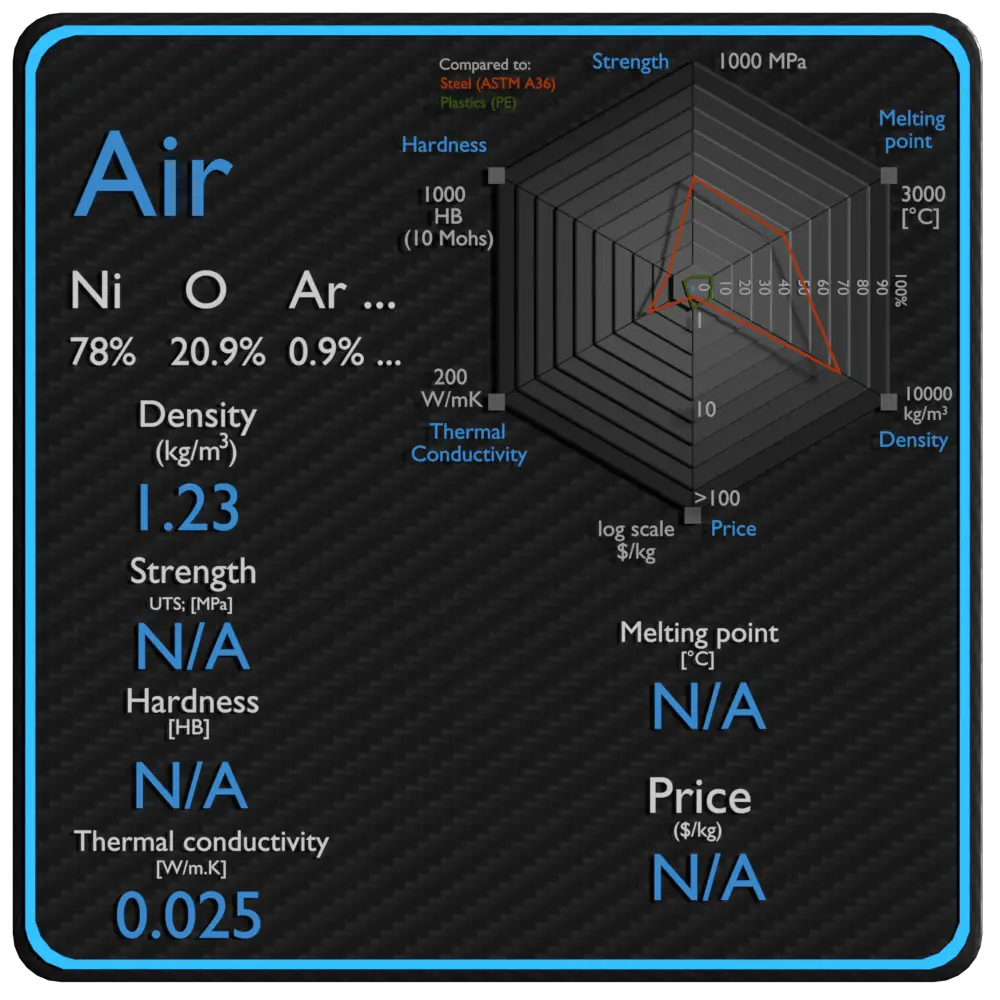
| Density | 1.23 kg/m3 |
| Ultimate Tensile Strength | N/A |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | N/A |
| Brinell Hardness | N/A |
| Melting Point | N/A |
| Thermal Conductivity | 0.025 W/mK |
| Heat Capacity | 1006 J/g K |
| Price | N/A |
Composition of Air
In engineering calculations, dry air is simplified to be a mixture of nitrogen, oxygen, and argon, and the corresponding mole fractions are 0.7812, 0.2096 and 0.0092, respectively. Air also contains a variable amount of water vapor, on average around 1% at sea level, and 0.4% over the entire atmosphere.
Applications of Air

Air is a natural resource and is available abundantly. It is an essential element of nature that support life on earth. Air is equally important for living organisms for their survival just like water. Air is very useful and has many applications. Uses of air are as follows: sustain life and growth, combustion, maintaining temperature, supplier of energy, photosynthesis. Air and other gases are generally good insulators, in the absence of convection. Therefore, many insulating materials (e.g.polystyrene) function simply by having a large number of gas-filled pockets which prevent large-scale convection. Alternation of gas pocket and solid material causes that the heat must be transferred through many interfaces causing rapid decrease in heat transfer coefficient.
Thermal Properties of Air
Air – Melting Point and Boiling Point
Melting point of Air is N/A.
Boiling point of Air is N/A.
Note that, these points are associated with the standard atmospheric pressure.
Air – Thermal Conductivity
Thermal conductivity of Air is 0.025 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
Coefficient of Thermal Expansion of Air
Linear thermal expansion coefficient of Air is N/A.
Thermal expansion is generally the tendency of matter to change its dimensions in response to a change in temperature. It is usually expressed as a fractional change in length or volume per unit temperature change.
Air – Specific Heat, Latent Heat of Fusion, Latent Heat of Vaporization
Specific heat of Air is 1006 J/g K.
Heat capacity is an extensive property of matter, meaning it is proportional to the size of the system. Heat capacity C has the unit of energy per degree or energy per kelvin. When expressing the same phenomenon as an intensive property, the heat capacity is divided by the amount of substance, mass, or volume, thus the quantity is independent of the size or extent of the sample.
Latent Heat of Fusion of Air is N/A.
Latent Heat of Vaporization of Air is N/A.
Latent heat is the amount of heat added to or removed from a substance to produce a change in phase. This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the gas (the pΔV work). When latent heat is added, no temperature change occurs. The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
Properties and prices of other materials
material-table-in-8k-resolution